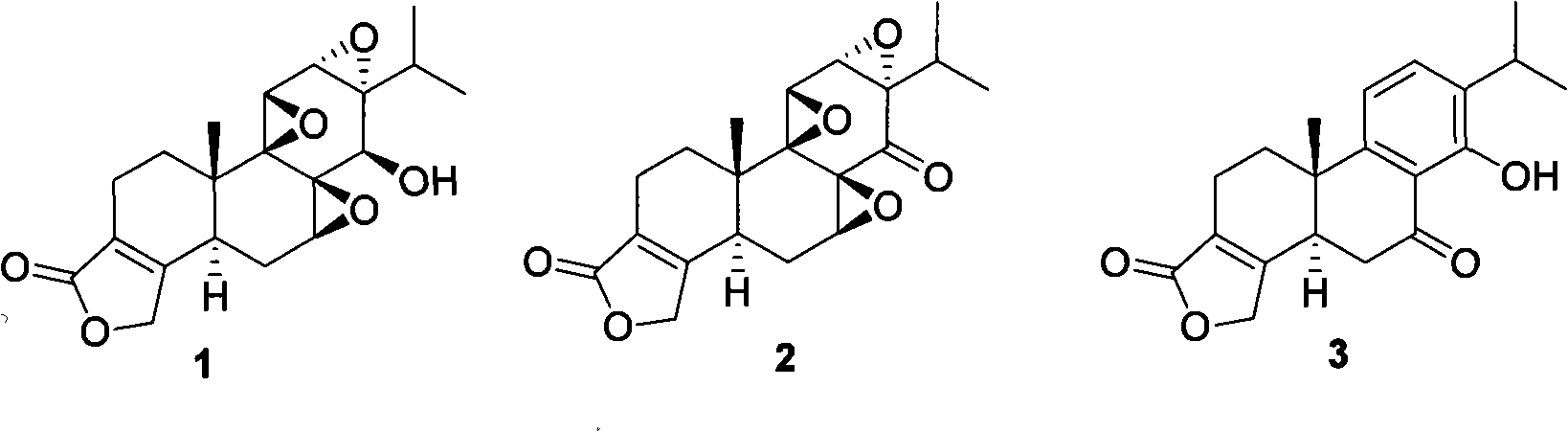
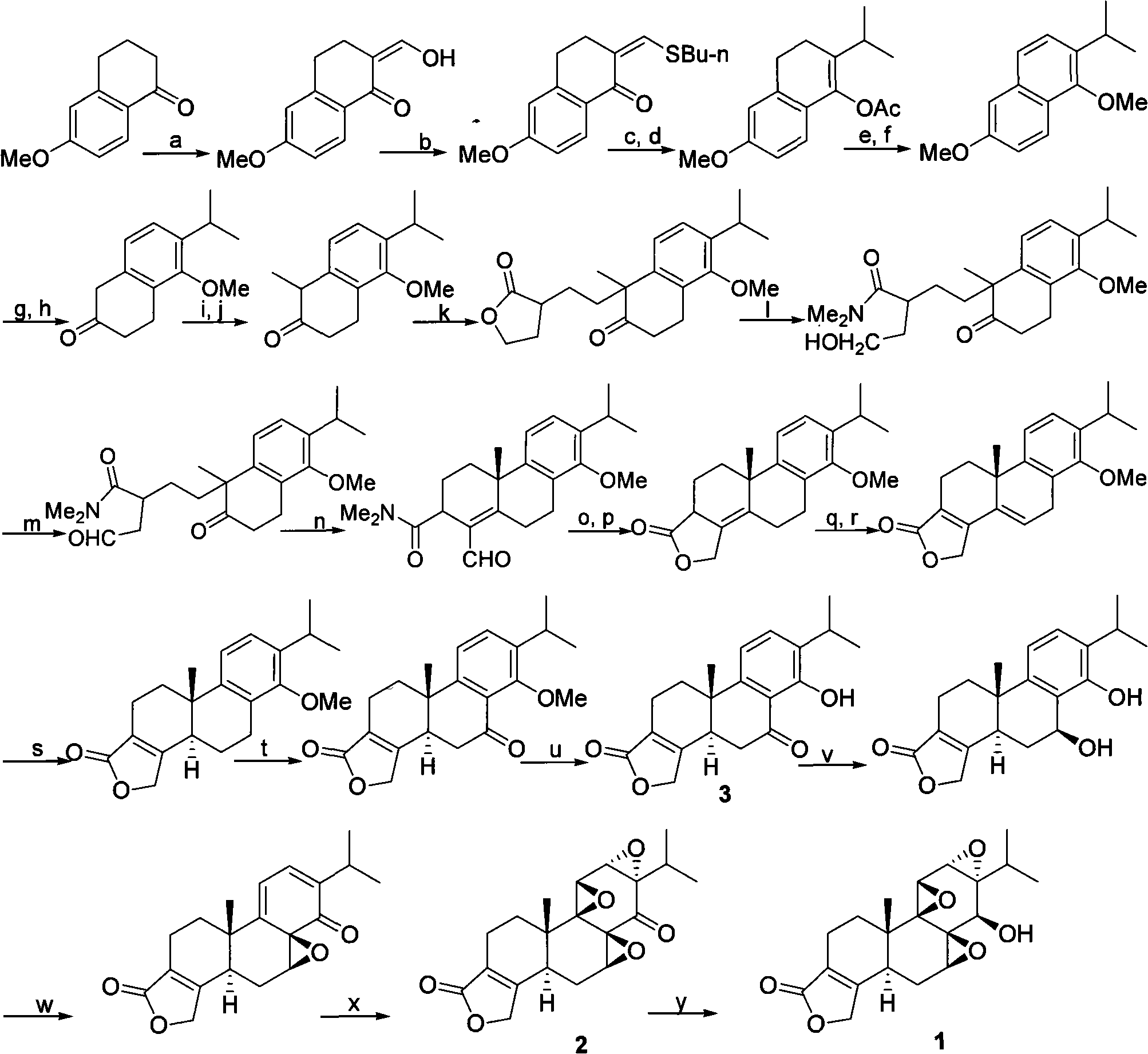
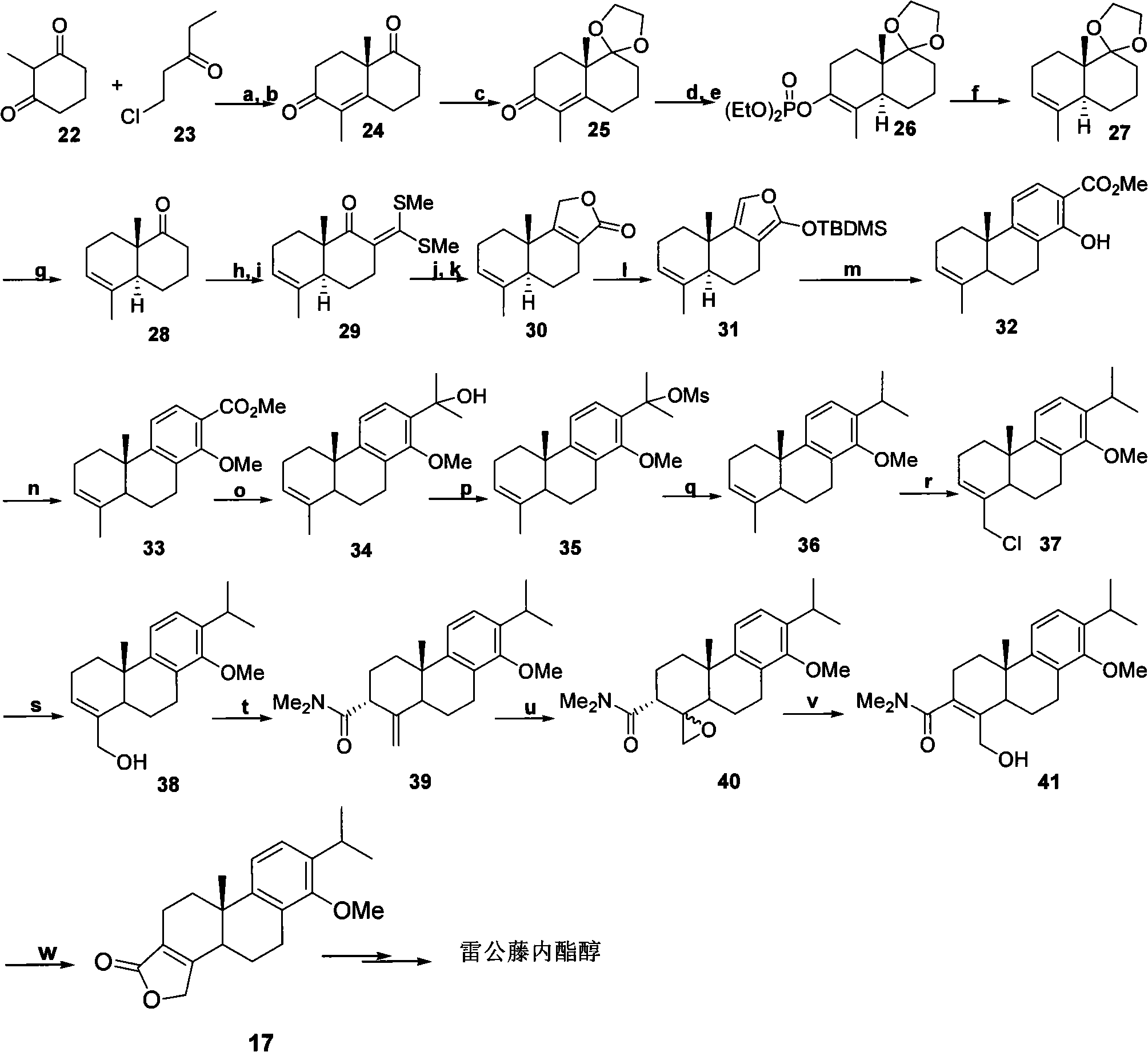
New composite method for triptolide
A technology for triptolide and a synthesis method, which is applied in the field of organic compound synthesis, can solve the problems of inability to synthesize a large number of target compounds, poor selectivity of asymmetric reaction, low yield of multi-step reaction, etc. The effect of technologicalization, short route and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] An embodiment of the present invention is provided below, wherein R 1 is methyl, R 2 For trimethylsilyl. The reaction of 4 and 5 was carried out in p-trifluoromethylbenzyl cinchonin bromide (formula 7) and sodium hydroxide, compound 9 was enol etherified with sodium hydride and trimethylchlorosilane and then reduced under Pd-C catalysis Compound 10 was obtained. Compound 14 was treated with sulfuric acid / isopropanol to give compound 3.
[0031] Formula 7
[0032] Ketone 4 (7.98g, 42mmol) iodide 5 (30g, 126mmol) and catalyst A (2.2g, 4.2mmol) were mixed and dissolved in toluene. 50% NaOH aqueous solution (6 mL) was added dropwise to the above solution with stirring at 0°C. After the dropwise addition, the reaction was slowly raised to room temperature and stirred for 48h. The organic layer was separated and the aqueous phase was extracted with ether. The organic phases were combined, the solvent was evaporated, and the residue was subjected to silica gel column ...
Embodiment 2
[0043] Specific embodiments of the present invention are provided below, wherein R 1 is ethyl, R 2 For triethylsilyl. The reaction of 4 and 5 is carried out in p-trifluoromethylbenzylquinine bromide (catalyst B) and sodium hydroxide, compound 9 is enol etherified with sodium hydride and triethylchlorosilane under Raney-Ni catalysis Reduction affords compound 10. Compound 14 was treated with methanesulfonic acid / isopropanol to give compound 3.
[0044] Ketone 4 (7.98 g, 42 mmol), iodide 5 (30 g, 126 mmol) and catalyst B (2.2 g, 4.2 mmol) were mixed and dissolved in toluene. 50% NaOH aqueous solution (6 mL) was added dropwise to the above solution with stirring at 0°C. After the dropwise addition, the reaction was slowly raised to room temperature and stirred for 48h. The organic layer was separated and the aqueous phase was extracted with ether. The organic phases were combined, the solvent was evaporated, and the residue was subjected to silica gel column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com