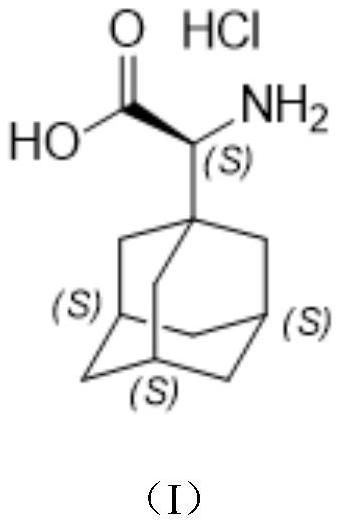
A kind of preparation method of (s)-2-(adamantan-1-yl)-2-aminoacetic acid hydrochloride
A technology of glycine hydrochloride and adamantane is applied in the preparation of organic compounds, the preparation of cyanide reactions, chemical instruments and methods, etc., and can solve problems such as being unsuitable for industrial production, unsafe, etc., and achieves convenient operation, The effect of short route and easy response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Chinese definitions of the abbreviations of the present invention: TLC: thin layer chromatography, DCM: dichloromethane, THF: tetrahydrofuran.
[0027] The reaction formula of embodiment 1 is as follows:
[0028]
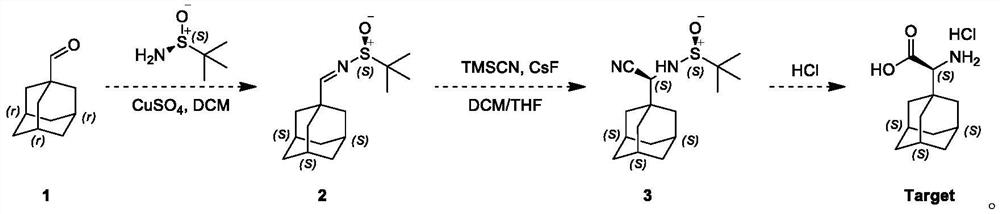
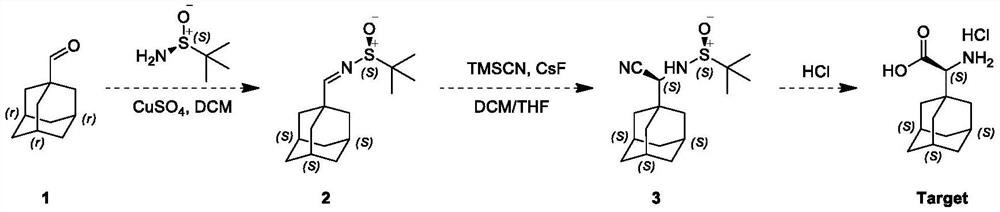
[0029] This embodiment adopts the following steps:
[0030] The first step: a 100 ml single-neck flask, magnetic stirring, to 1-adamantanecarboxaldehyde, namely compound 1 (1.64g, 9.87mmol) and tert-butylsulfinamide (1.21g, 10.85mmol) and CuSO 4 To a solution of (copper sulfate 3.19 g, 19.53 mmol) in dichloromethane (20 mL) was added pyridine p-toluenesulfonate (125 mg, 0.494 mmol), and the resulting reaction mixture was stirred at 20° C. for 12 hours under nitrogen protection; TLC (petroleum ether / ethyl acetate = 3 / 1, Rf = 0.2, phosphomolybdic acid) indicated complete consumption of compound 1 with the formation of a new spot; the reaction mixture was filtered, washed with saturated NaHCO 3 solution (10 mL of sodium bicarbonate solution) and saturated a...
Embodiment 2
[0034] Chinese definitions of the abbreviations of the present invention: TLC: thin layer chromatography, DCM: dichloromethane, THF: tetrahydrofuran.
[0035] The reaction formula of embodiment 2 is as follows:
[0036]
[0037] This embodiment adopts the following steps:
[0038] The first step: a 100 ml single-neck flask, magnetic stirring, to 1-adamantanecarboxaldehyde, namely compound 1 (1.64g, 9.87mmol) and tert-butylsulfinamide (1.21g, 10.85mmol) and CuSO 4 To a solution of (copper sulfate 6.39 g, 39.05 mmol) in dichloromethane (20 mL) was added pyridine p-toluenesulfonate (500 mg, 1.974 mmol), and the resulting reaction mixture was stirred under nitrogen at 22.5°C for 14 hours; TLC (petroleum ether / ethyl acetate = 3 / 1, Rf = 0.2, phosphomolybdic acid) indicated complete consumption of compound 1 with the formation of a new spot; the reaction mixture was filtered, washed with saturated NaHCO 3 solution (10 mL of sodium bicarbonate solution) and saturated ammonium chl...
Embodiment 3
[0042] Chinese definitions of the abbreviations of the present invention: TLC: thin layer chromatography, DCM: dichloromethane, THF: tetrahydrofuran.
[0043] The reaction formula of embodiment 3 is as follows:
[0044]
[0045] This embodiment adopts the following steps:
[0046] The first step: a 100 ml single-neck flask, magnetic stirring, to 1-adamantanecarboxaldehyde, namely compound 1 (1.64g, 9.87mmol) and tert-butylsulfinamide (1.21g, 10.85mmol) and CuSO 4 To a solution of (copper sulfate 4.79 g, 29.29 mmol) in dichloromethane (20 mL) was added pyridine p-toluenesulfonate (250 mg, 0.987 mmol), and the resulting reaction mixture was stirred under nitrogen at 25°C for 16 hours; TLC (petroleum ether / ethyl acetate = 3 / 1, Rf = 0.2, phosphomolybdic acid) indicated complete consumption of compound 1 with the formation of a new spot; the reaction mixture was filtered, washed with saturated NaHCO 3 solution (10 mL of sodium bicarbonate solution) and saturated ammonium chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com