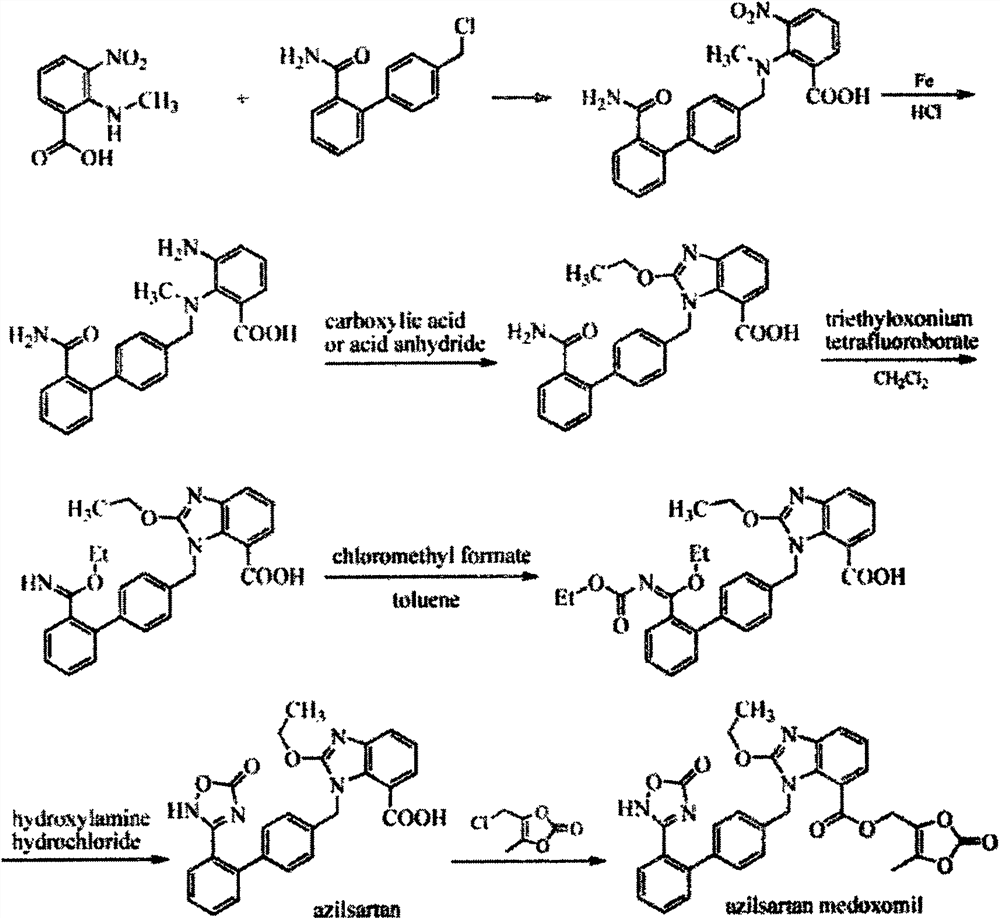
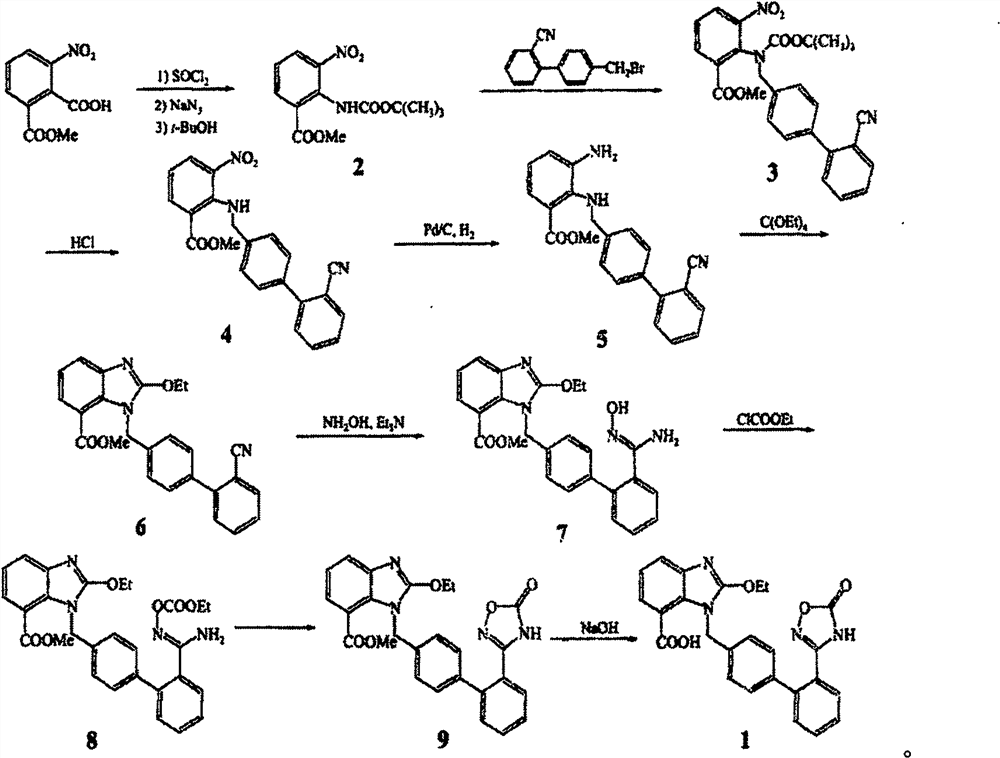
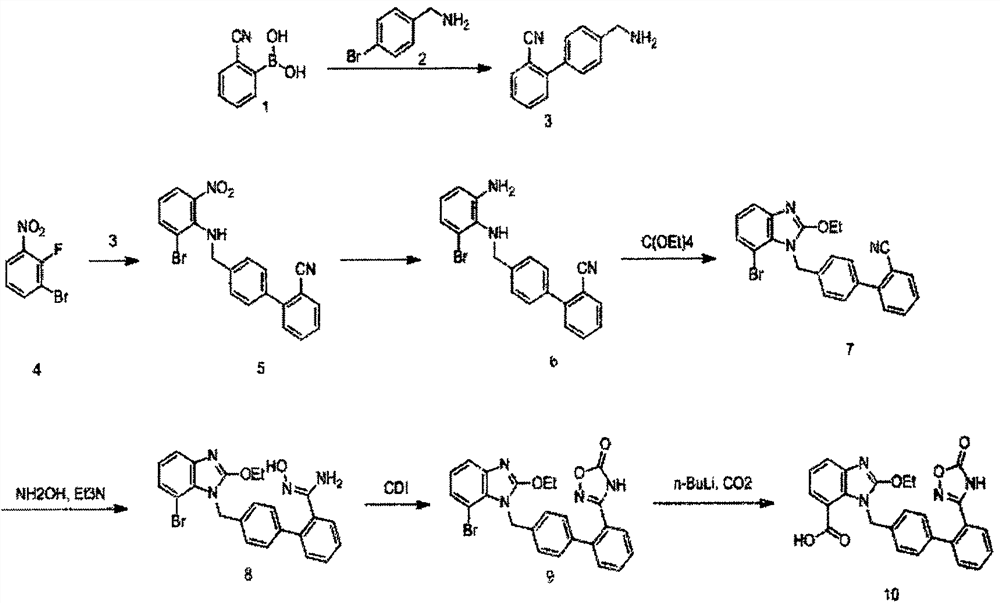
A new preparation process of azilsartan
A technology of compound and synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of long steps and limitations of industrial production, and achieve the effect of less by-products, short method route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthesis of embodiment 1. compound 3:
[0021] Compound 1 (7.4g, 40mmol), compound 2 (5.9g, 40mmol), Na 2 CO 3 (2M, 80 mmol, ), Pd(dppf)Cl 2 (2mmol) and 150mL of ethylene glycol dimethyl ether were added successively in a 500mL flask under a nitrogen atmosphere, refluxed for 2 hours, then extracted with ethyl acetate, NaHCO3 solution, brine were washed successively, dried over anhydrous magnesium sulfate, filtered, The crude product obtained by concentration was directly used in the next step without purification.
Embodiment 2
[0022] The synthesis of embodiment 2. compound 5:
[0023] Compound 4 (9.0g, 41mmol), Na 2 CO 3 (8.7g, 82mmol), compound 3 (8.1g, crude product) and 150mL of DMF were added to a 250mL flask, reacted at 130°C for 2h, cooled, concentrated, diluted with water, extracted with ethyl acetate, dried, and purified by column chromatography 5 (13g, yield, 80%)
Embodiment 3
[0024] The synthesis of embodiment 3. compound 6:
[0025] SnCl 2 (10g, 54mmol) was added in the ethyl acetate (300mL) solution of compound 5 (13g, 32mmol), then refluxed for 17h, after monitoring that the reaction was complete, direct concentration column chromatography purified to obtain compound 6 (10.5g, yield 87%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com