Preparation method of 5,8-dimethoxy-2-tetralin ketone
A technology of dimethoxy and tetralone, applied in 5 fields, can solve problems such as difficult preparation, and achieve the effects of easy purification, mild reaction conditions and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
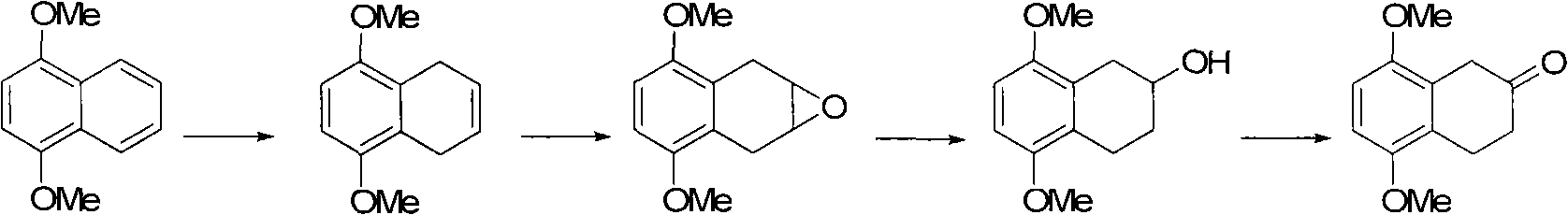
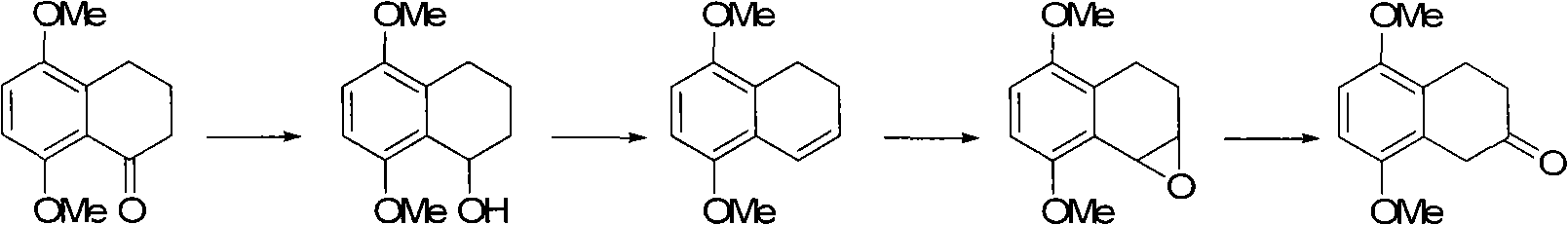
Image
Examples
Embodiment 1
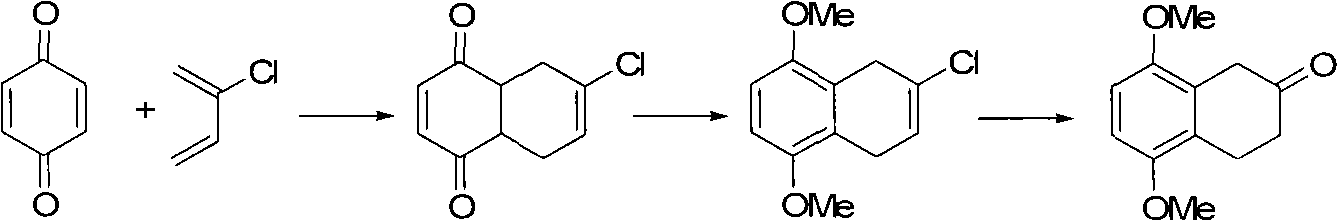
[0032] Dissolve 25 grams of p-benzoquinone in 45 milliliters of acetic acid, and under stirring, pass 38 grams of butadiene gas into the reaction solution within 1 hour at 10±5 degrees Celsius. After the gas is passed, continue to stir until the reaction lasts for 10 hours Finally, the reaction solution was poured into 250 ml of ice water, extracted three times with 200 ml of ether, the combined organic phases were washed with saturated sodium bicarbonate in turn, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and evaporated to dryness, and the residue was washed with silica gel Purified by column chromatography to obtain 30 g of 4a,5,8,8a-tetrahydro-1,4-naphthoquinone with a yield of 80%.
[0033] Suspend 25.3 grams of 4a, 5, 8, 8a-tetrahydro-1, 4-naphthoquinone in 120 grams of water, add 33.6 grams of KOH, heat and reflux and stir for 3 hours, then slowly drop 57 grams of disulfuric acid into the reaction solution After the addition of methyl este...
Embodiment 2
[0038] Dissolve 50 grams of p-benzoquinone in 100 milliliters of acetic acid, under stirring, at 10 ± 5 degrees Celsius, pass 70 grams of butadiene gas into the reaction solution within 3 hours, after the gas is passed, continue to stir until the reaction lasts for 10 hours Finally, the reaction solution was poured into 500 ml of ice water, and filtered to obtain 55 g of 4a,5,8,8a-tetrahydro-1,4-naphthoquinone with a yield of 77%.
[0039] Suspend 40 grams of 4a, 5, 8, 8a-tetrahydro-1, 4-naphthoquinone in 200 grams of water, add 120 grams of KOH, heat to reflux and stir for 12 hours, then slowly drop 85 grams of methyl iodide into the reaction solution After the addition, continue to heat and reflux for 5 hours, cool the reaction solution to room temperature, extract three times with 100 ml of n-hexane, wash the combined organic phases with water and saturated brine, dry over anhydrous sodium sulfate, filter and evaporate to dryness, The residue was purified by silica gel colu...
Embodiment 3
[0042] Dissolve 22 kg of benzoquinone in 50 kg of acetic acid, under stirring, below 25 degrees Celsius, feed 12 kg of butadiene gas into the reaction solution within 15 hours, then continue to stir for 10 hours, and pour the reaction solution into 250 kg After stirring in ice water for 1 hour, filter by centrifuge, wash the filter cake with 200 kg of water, and air-dry to obtain 26 kg of 4a,5,8,8a-tetrahydro-1,4-naphthoquinone with a yield of 80%.
[0043] Suspend 26 kilograms of 4a, 5, 8, 8a-tetrahydro-1,4-naphthoquinone in 160 kilograms of water, add 28 kilograms of KOH, heat and reflux and stir for 3 hours, then slowly drop 50 kilograms of sulfuric acid disulfide into the reaction solution Methyl ester, continue to react for 10 hours after the addition, cool the reaction solution to room temperature, pour it into 500 kg of ice water, stir for 1 hour, filter, wash the filter cake with 200 kg of water, and dry it to obtain 5,8-dimethyl ester 23 kg of oxy-1,4-dihydronaphthale...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com