Opioids composition for treating withdrawal symptoms of morphine addiction
A technology for opioids and withdrawal symptoms, which is applied in the field of opioids and clinical detoxification substitution therapy, can solve problems such as aggravation, obvious withdrawal reaction, secondary addiction, etc., so as to improve the quality of life and avoid secondary addiction. , the effect of reducing withdrawal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Morphine: 10-100mg / kg
[0022] Buprenorphine: 0.4mg / kg
[0023] Dezocine: 0.4mg / kg
[0024] Mixed solution A: buprenorphine: pentazocine = 1:1, that is, buprenorphine and pentazocine are 0.2mg / kg and 0.2mg / kg respectively, dissolved in DMSO and diluted to 1ml, passed through the aperture After filtering with 0.22μM filter paper, subpackage, seal, sterilize and store at 4°C, and freezing is strictly prohibited.
[0025] Mixed solution B: buprenorphine: tramadol = 1:1, that is, buprenorphine and tramadol are 0.2mg / kg and 0.2mg / kg respectively, dissolved in DMSO and diluted to 1ml, passed through a pore size of 0.22μM After filtering with filter paper, subpackage, seal, sterilize and store at 4°C. Freezing is strictly prohibited.
[0026] Mixed solution C: buprenorphine: dezocine = 1:1, that is, buprenorphine and dezocine are 0.2mg / kg and 0.2mg / kg respectively, dissolved in DMSO and diluted to 1ml, passed through a pore size of 0.22μM After filtering with filter paper,...
Embodiment 2
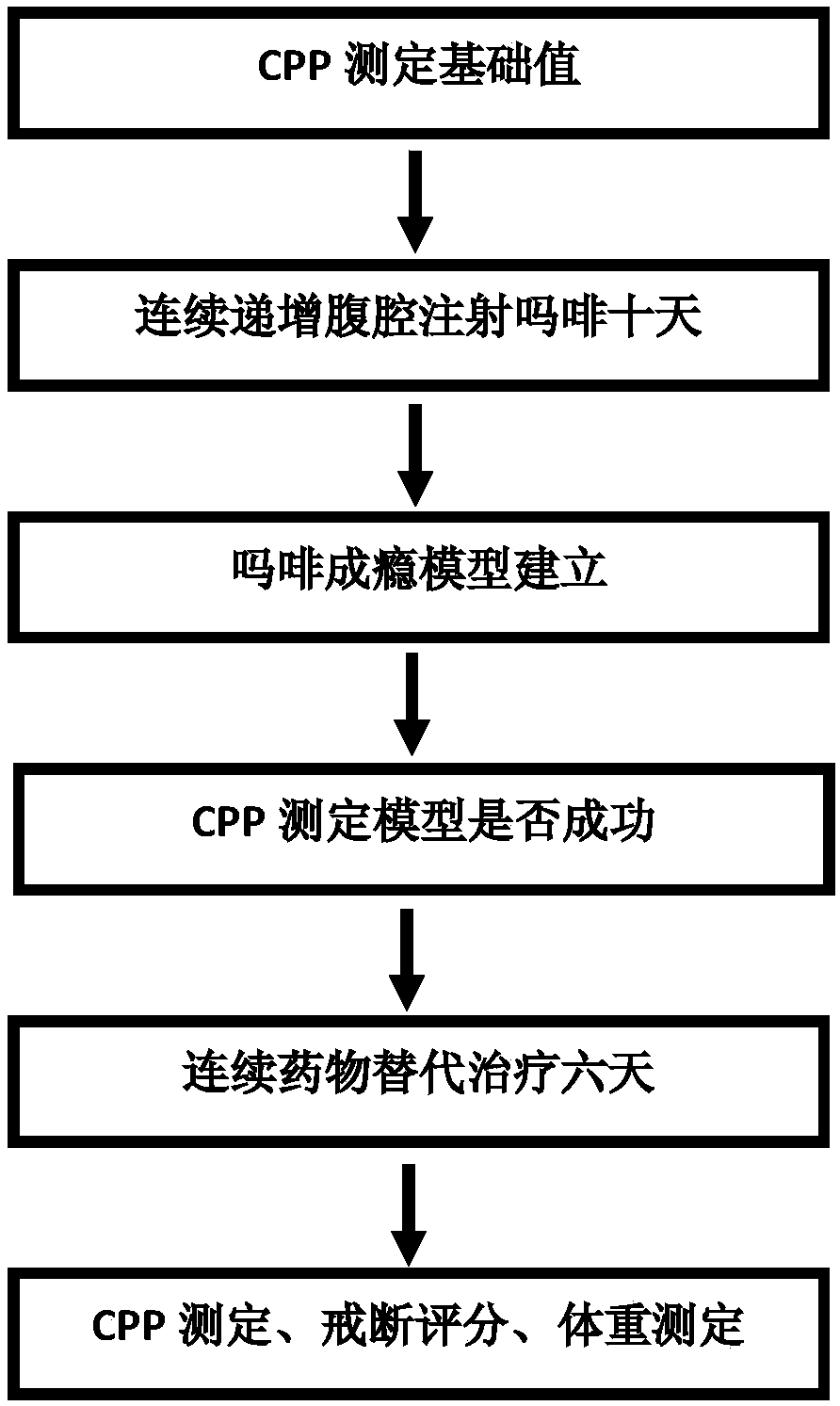
[0035] After the successful establishment of the morphine addiction model, the addicted rats were randomly divided into 11 groups, 20 in each group. The blank control group was given the same dose of normal saline or natural withdrawal, and the other groups were given buprenorphine and dezocine respectively. , mixed solution A, mixed solution B, mixed solution C, mixed solution D, mixed solution E, mixed solution F, CPP was measured after six consecutive days of replacement therapy. The results are shown in Table 2. The white box residence time of rats after addiction was significantly higher than that before addiction, indicating that the morphine addiction model was successful. The replacement therapy in the experimental group can significantly reduce the CPP value of morphine-addicted rats (P<0.05), but the improvement effect of mixture A and mixture B on CPP is not as good as that of buprenorphine alone, and the therapeutic effect of mixture E is obvious It is worse than ot...
Embodiment 3
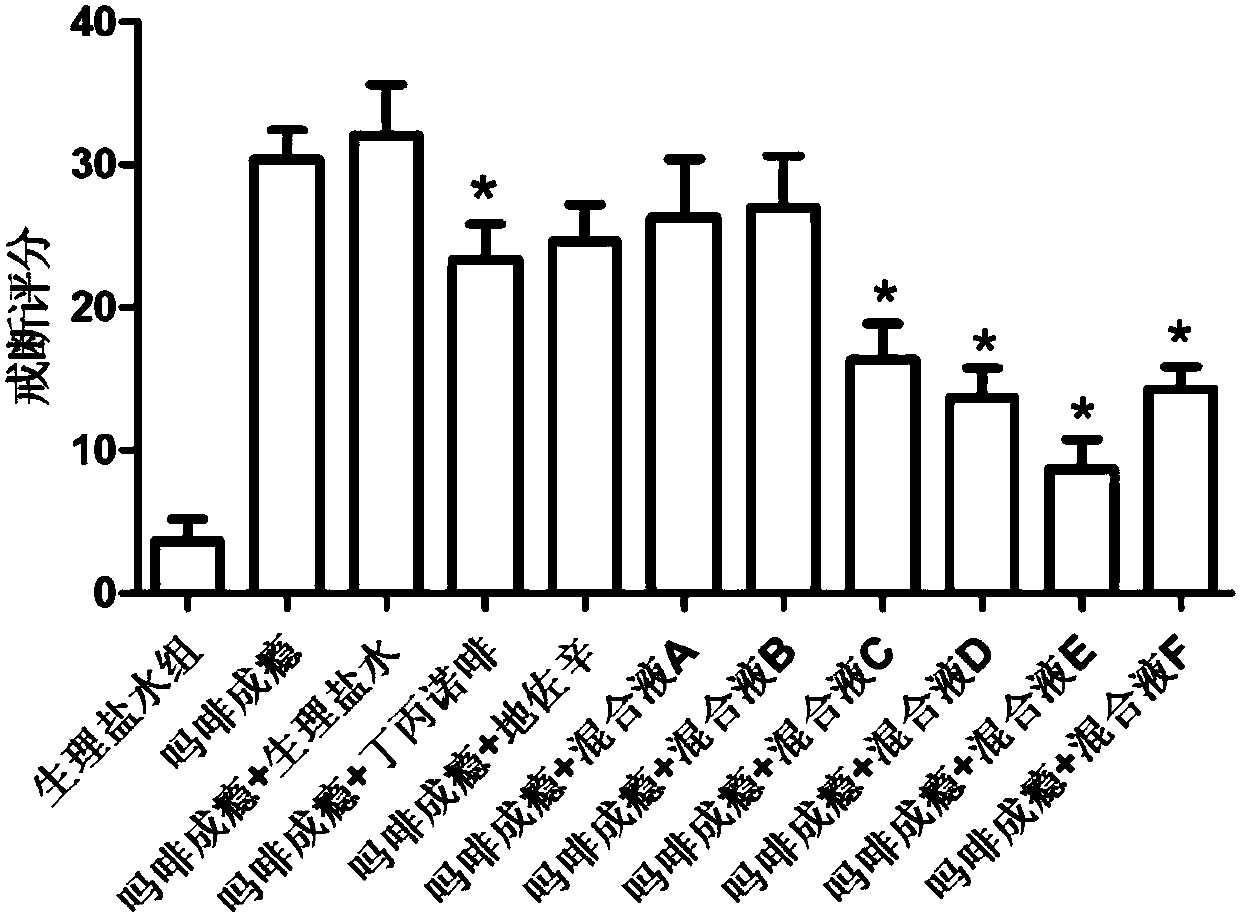
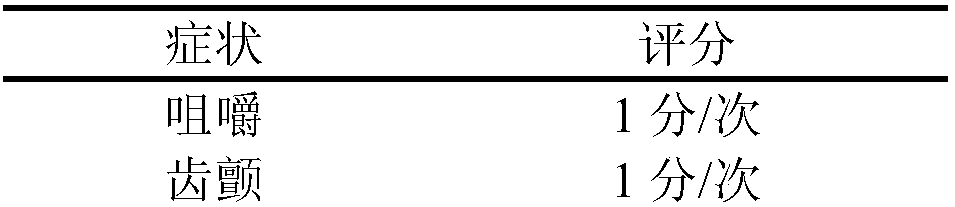
[0040] After the successful establishment of the morphine addiction model, the addicted rats were randomly divided into 11 groups, 20 in each group. The blank control group was given the same dose of normal saline or natural withdrawal, and the other groups were given buprenorphine and dezocine respectively. , Mixed Solution A, Mixed Solution B, Mixed Solution C, Mixed Solution D, Mixed Solution E, Mixed Solution F, the withdrawal score was performed after six consecutive days of substitution therapy. The result is as figure 2 As shown, after the replacement treatment of the experimental group, the effect of mixed solution A and mixed solution B on improving withdrawal symptoms was not as good as that of buprenorphine alone, and the withdrawal symptoms of rats in the combined use of buprenorphine and dezocine group were significantly reduced ( P<0.05), and the withdrawal symptoms of the mixture E are the most significant, indicating that the combined use of buprenorphine, pen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com