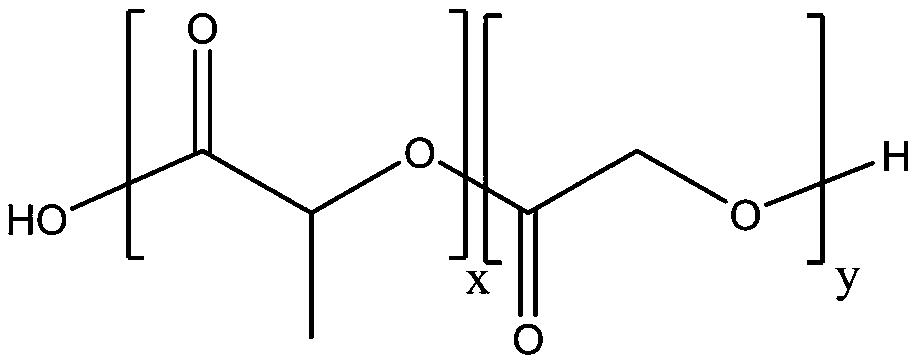
A drug sustained-release preparation containing dezocine and its preparation method
A sustained-release preparation, the technology of dezocine, applied in the field of medicine, achieves the effect of relieving pain and stabilizing drug release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Step a. At room temperature, weigh 120 mg of dezocine and 0.1 g polylactic acid-glycolic acid block copolymer (molecular weight is 100 KDa, intrinsic viscosity is 0.3 dl / g) and 0.1 g poloxamer 188 by high The shear dispersing emulsifier is dispersed in ethanol and configured as an organic phase solution. The stirring speed of the high-speed shearing machine is 5000rpm, and the stirring time is 30min;
[0068] Step b. Weighing 0.1g sodium chloride as the osmotic pressure regulator and 0.1g wetting agent are dissolved in the water for injection of 100mL to form the inner aqueous phase solution and the outer aqueous phase solution respectively;
[0069] Step c. using a buffer solution of sodium dihydrogen phosphate / sodium hydrogen phosphate to adjust the pH value of the inner aqueous phase solution obtained in step b to 2-7;
[0070] Step d. At room temperature, mix the organic phase solution obtained in the above step a and the 20mL internal aqueous phase solution obtaine...
Embodiment 7
[0079] Example 7 Study on the Analgesic Effect of Dezocine Sustained-release Preparation Take 30 male SD rats, weighing (180±20) g, and place them in the experimental environment for 3 days.
[0080] Place the mouse on a metal plate with a temperature of 60°C, and stimulate the foot of the mouse to produce a pain response. When the mouse licks the foot, it will produce pain. Choose the mouse that licks the foot for less than 20 seconds after touching the hot plate. Mice were used in the experiment, and the ones longer than 20s were eliminated, and the average value was taken for three times, and 9 mice with the average value less than 20s were randomly selected for the experiment, and were divided into 3 groups on average, with 3 mice in each group.
[0081] Administration method:
[0082] Test group: administration injection dezocine injection 0.2 μg / 0.2mL physiological saline of embodiment 1 of the present invention;
[0083] Control group: 0.2 mL of Dezocine Injection (Yan...
Embodiment 8
[0089] Example 8 Pharmacokinetic evaluation of dezocine sustained-release preparation in rats Take 30 male SD rats with a body weight of (180±20) g, and place them in the experimental environment to raise them for 3 days. Fasting for 12 hours before the experiment, the dezocine preparation prepared by the method described in Example 1 of the present invention and the administration injection control sample dezocine injection (Yangzijiang Pharmaceutical Group Co., Ltd. Company, batch number: 12030121 Specification: 1 mL: 5 mg) 0.2 mL. After 30 minutes, 1, 2, 3, 4, 5, 6, 7, and 8 hours, take about 0.2 mL of blood from the ear vein, put it in a pre-heparinized 1.5 mL centrifuge tube, centrifuge at 5000 rpm for 5 minutes, and draw The upper layer of plasma was stored frozen at -80°C.
[0090] At room temperature, accurately draw 100 μL of plasma sample into a 5 mL centrifuge tube, add 100 μL of saturated sodium bicarbonate, mix well, add 2 mL of ethyl acetate, continue vortex mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com