Dezocine A crystal form and preparation method thereof
A technology of dezocine and crystal form, applied in the field of dezocine A crystal form and its preparation, can solve problems such as no crystal form involved, and achieve the effects of reducing product impurity content, good reproducibility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this embodiment, the method for preparing crystal form of dezocine A includes the following steps:
[0037] 1) Dissolve 10 g of crude dezocine in 100 ml of methanol, heat to reflux for 30 minutes, and filter while hot;
[0038] 2) Cool down to -5°C to charge the analytical crystals, filter and dry to obtain crude dezocine; obtain 6.5g off-white or white crystalline powder, with a yield of 65%.
[0039] Take the off-white crystalline powder obtained in Example 1 for structural analysis, and the results are as follows:
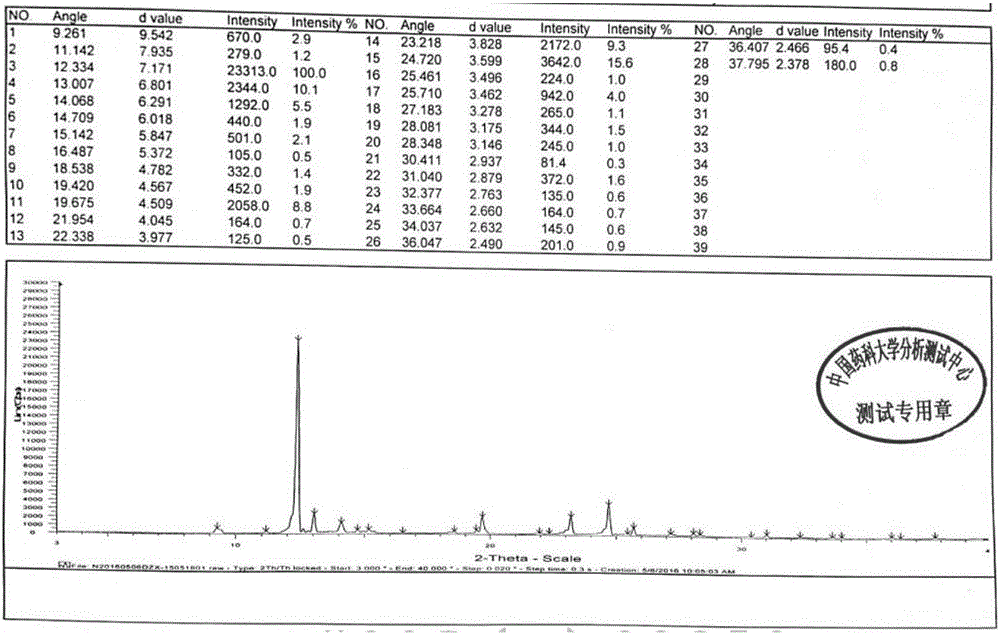
[0040] Its X-ray powder diffraction pattern is as follows figure 1 Shown.
[0041] The elemental analysis results were: C (%): 78.32, N (%): 5.70, H (%): 9.41, O (%): 6.52.
[0042] Its infrared spectrum (IR) diagram is as figure 2 Shown.
[0043] Its differential scanning thermal analysis chart (DSC chart) is as image 3 Shown.
[0044] The thermogravimetric (TG) diagram of Dezocine A crystal form is as follows Figure 4 Shown.
[0045] The HPLC detection method i...
Embodiment 2
[0049] In this embodiment, the method for preparing crystal form of dezocine A includes the following steps:
[0050] 1) Dissolve 10g of crude dezocine in 200ml of ethanol, heat to reflux for 30 minutes, and filter while hot;
[0051] 2) Cool down to 30°C to charge the analytical crystals, filter, and dry to obtain crude dezocine; obtain 7.5 g of off-white crystalline powder with a yield of 75%.
[0052] The structure analysis result of the product of Example 2 is not significantly different from the structure analysis result of Example 1.
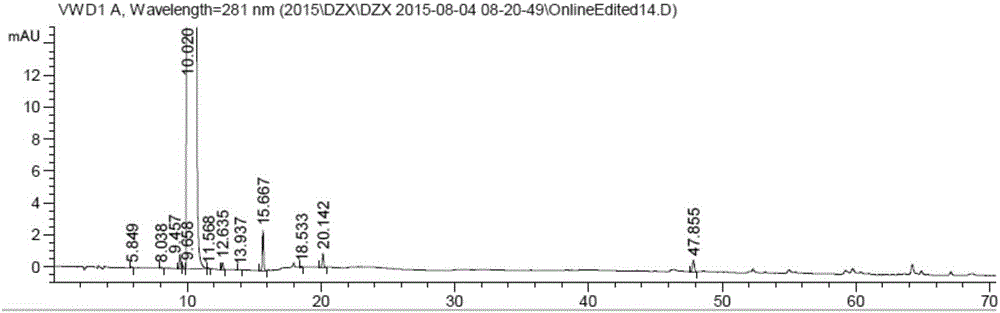
[0053] The HPLC detection method is as in Example 1, HPLC diagram and Figure 5 Similar, no obvious difference, the enantiomer is not more than 0.2%.
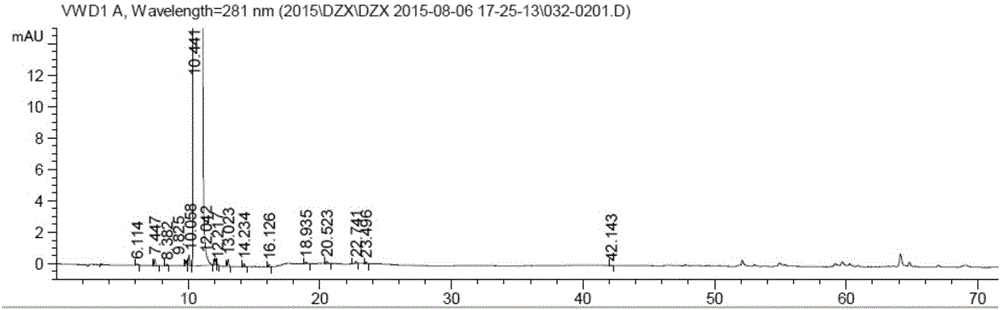
[0054] The related substance detection method is as in Example 1. The related substance detection spectrum is Image 6 Show similar. Single impurity is not more than 0.05%, total impurity is not more than 0.3%.
Embodiment 3
[0056] In this embodiment, the method for preparing crystal form of dezocine A includes the following steps:
[0057] 1) Dissolve 10g of crude dezocine with 150ml of ethanol, heat to reflux for 30 minutes, and filter while hot;
[0058] 2) Cool down to 5-10°C to charge the analytical crystals, filter, and dry to obtain crude dezocine; obtain 8 g of off-white crystalline powder, with a yield of 80%.
[0059] The structure analysis result of the product of Example 3 is not significantly different from the structure analysis result of Example 1.
[0060] The HPLC detection method is as in Example 1, HPLC diagram and Figure 5 Similar, no obvious difference, the enantiomer is not more than 0.2%.
[0061] The related substance detection method is as in Example 1. The related substance detection spectrum is Image 6 Show similar. Single impurity is not more than 0.05%, total impurity is not more than 0.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com