A compound drug sustained-release preparation containing dezocine and lidoine and its preparation method
A sustained-release preparation, the technology of dezocine, applied in the field of medicine, can solve the problems of reducing the frequency of dosing, poor bioavailability of lidocaine, etc., and achieve the effect of relieving pain and stabilizing the drug release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
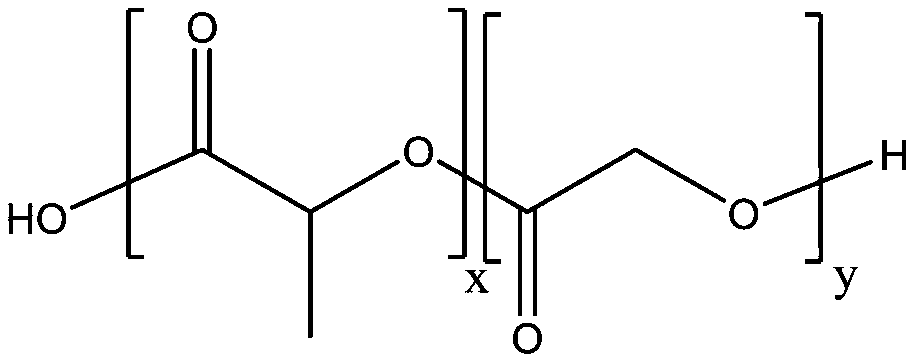
[0071] Step a. At room temperature, weigh 120 mg of dezocine and 0.1 g polylactic acid-glycolic acid block copolymer (molecular weight is 100 KDa, intrinsic viscosity is 0.3 dl / g) and 0.1 g poloxamer 188 by The high-shear dispersing emulsifier is dispersed in ethanol and configured as an organic phase solution. The stirring rate of the high-speed shearing machine is 5000rpm, and the stirring time is 30min;
[0072] Step b. the lidocaine that takes 120mg is dissolved in 100mL water for injection, forms inner water phase, adopts the buffer solution of sodium dihydrogen phosphate / sodium hydrogen phosphate that the pH value of inner water phase solution is adjusted to 2-7;
[0073] Step c. Weighing 0.1g sodium chloride as an osmotic pressure regulator and 0.1g wetting agent are dissolved in 100mL of water for injection to form an external aqueous phase solution;
[0074] Step d. At room temperature, mix the organic phase solution obtained in step a above with the internal aqueous ...
Embodiment 2
[0078] Step a. At room temperature, weigh 150 mg of dezocine and 0.2 g polylactic acid-glycolic acid block copolymer (molecular weight is 100 KDa, intrinsic viscosity is 0.3 dl / g) and 0.1 g poloxamer 188 by The high-shear dispersing emulsifier is dispersed in ethanol and configured as an organic phase solution. The stirring rate of the high-speed shearing machine is 5000rpm, and the stirring time is 30min;
[0079] Step b. the lidocaine that takes 150mg is dissolved in 100mL water for injection, forms inner water phase, adopts the buffer solution of sodium dihydrogen phosphate / sodium hydrogen phosphate that the pH value of inner water phase solution is adjusted to 2-7;
[0080] Step c. Weighing 0.1g sodium chloride as an osmotic pressure regulator and 0.1g wetting agent are dissolved in 100mL of water for injection to form an external aqueous phase solution;
[0081] Step d. At room temperature, mix the organic phase solution obtained in step a above with the internal aqueous ...
Embodiment 3
[0085] Step a. At room temperature, weigh 120 mg of dezocine and 0.1 g polylactic acid-glycolic acid block copolymer (molecular weight is 100 KDa, intrinsic viscosity is 0.3 dl / g) and 0.15 g poloxamer 188 by The high-shear dispersing emulsifier is dispersed in ethanol and configured as an organic phase solution. The stirring rate of the high-speed shearing machine is 5000rpm, and the stirring time is 30min;
[0086] Step b. the lidocaine that takes 120mg is dissolved in 100mL water for injection, forms inner water phase, adopts the buffer solution of sodium dihydrogen phosphate / sodium hydrogen phosphate that the pH value of inner water phase solution is adjusted to 2-7;
[0087] Step c. Weighing 0.1g sodium chloride as an osmotic pressure regulator and 0.1g wetting agent are dissolved in 100mL of water for injection to form an external aqueous phase solution;
[0088] Step d. At room temperature, mix the organic phase solution obtained in step a above with the internal aqueous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com