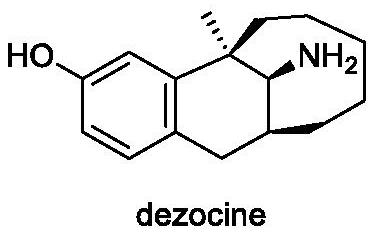
Asymmetric synthesis method of dezocine key intermediate
A synthesis method and intermediate technology, which is applied in the field of synthesis of key intermediates of dezocine, can solve the problems of low catalyst stereoselectivity, troublesome reaction process, high price, etc., achieve good yield, simple operation, and high preparation method concise effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
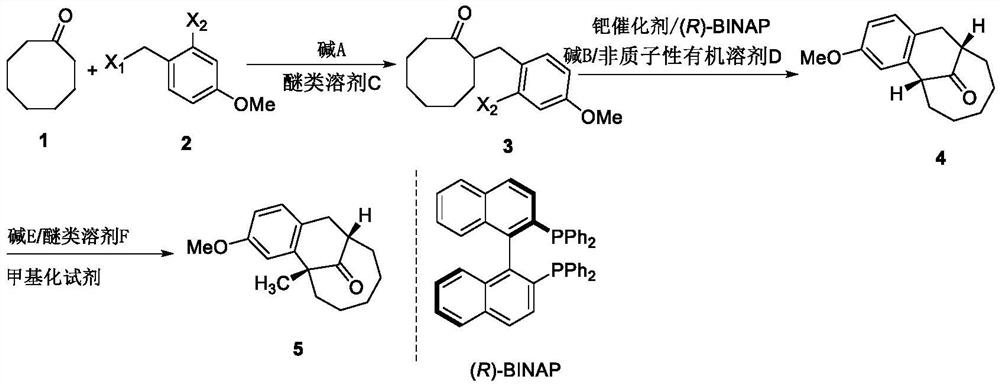
[0039] A kind of asymmetric synthetic method of dezocine key intermediate, synthetic route is as follows:
[0040]
[0041](1) Preparation of compound 3
[0042] Under the condition of a dry ice-ethanol bath, control the internal temperature below -60°C, slowly add lithium diisopropylamide solution LDA (16.5mL, 33.0mmol, 2M in THF / Heptane), the addition was completed in about 15min, and the resulting solution was stirred at low temperature for 1h. Afterwards, a THF solution (25 mL) of compound 2 (10 g, 36.0 mmol; refer to the preparation method in Org. Bio. Chem. 2007, 5, 143-150) was added to the reaction system. The system naturally returned to room temperature and reacted for 3h. After the reaction is over, add saturated ammonium chloride solution to the reaction system to quench the reaction, then add ethyl acetate, separate the organic phase, extract the aqueous phase twice with ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, and filter ...
Embodiment 2
[0057] An asymmetric synthesis method of a key intermediate of dezocine, which is the same as the synthetic route of Example 1, the difference is that the preparation conditions of compound 3 are different, and the preparation of compound 3 is specifically as follows:
[0058] Under the condition of a dry ice-ethanol bath, the internal temperature was controlled below -60°C, and slowly added bis(trimethylsilyl)amide lithium LiHMDS ( 22mL, 22.0mmol, 1M inTHF), the addition was completed in about 15min, and the resulting solution was stirred at low temperature for 1h. After that, a tetrahydrofuran solution (15 mL) of compound 2 (6.72 g, 24.0 mmol) was added to the reaction system. The system naturally returned to room temperature and reacted for 3h. After the reaction is over, add saturated ammonium chloride solution to the reaction system to quench the reaction, then add ethyl acetate, separate the organic phase, extract the aqueous phase twice with ethyl acetate, combine the ...
Embodiment 3
[0060] An asymmetric synthesis method of a key intermediate of dezocine, which is the same as the synthetic route of Example 1, the difference is that the preparation conditions of compound 3 are different, and the preparation of compound 3 is specifically as follows:
[0061] Under the conditions of a dry ice-ethanol bath, control the internal temperature below -60°C, slowly add lithium diisopropylamide solution to cyclooctanone 2 (2.0g, 15.8mmol) in methyltetrahydrofuran (MeTHF) solution (30mL) LDA (8.7mL, 17.4mmol, 2Min THF / Heptane) was added in about 15min, and the resulting solution was stirred at low temperature for 1h. After that, a solution (15 mL) of compound 2 (5.32 g, 19.0 mmol) in methyl tetrahydrofuran was added to the reaction system. The system naturally returned to room temperature and reacted for 3h. After the reaction is over, add saturated ammonium chloride solution to the reaction system to quench the reaction, then add ethyl acetate, separate the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com