Biomarker for severe asthma and application thereof
A technology for severe asthma, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems of severe asthma epidemiology, genetic research pathological characteristics and pathogenesis are not completely clear, and achieve early realization Effects of diagnosis and mortality reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Screening Gene Markers Related to Asthma
[0061] 1. Sample collection
[0062] 3 ml of peripheral venous blood was collected from 3 normal persons, 3 normal adult asthmatics, and 3 severe adult asthmatics. All patients gave informed consent, and all the above specimens were obtained with the consent of the ethics committee.
[0063]Patient Inclusion Criteria:
[0064] Ordinary asthma patients meet the diagnostic criteria for bronchial asthma in the 2016 guidelines for the prevention and treatment of bronchial asthma; severe asthma meets the "Chinese Expert Consensus on the Diagnosis and Treatment of Severe Asthma"; the three groups of samples are all taking any drugs in a short period of time; (4) Age > 18 years old .
[0065] Patient Exclusion Criteria:
[0066] Patients with co-infection, pulmonary embolism, chronic bronchitis, tuberculosis and blood system diseases; patients with abnormal liver function.
[0067] 2. RNA sample preparation and quality a...
Embodiment 2
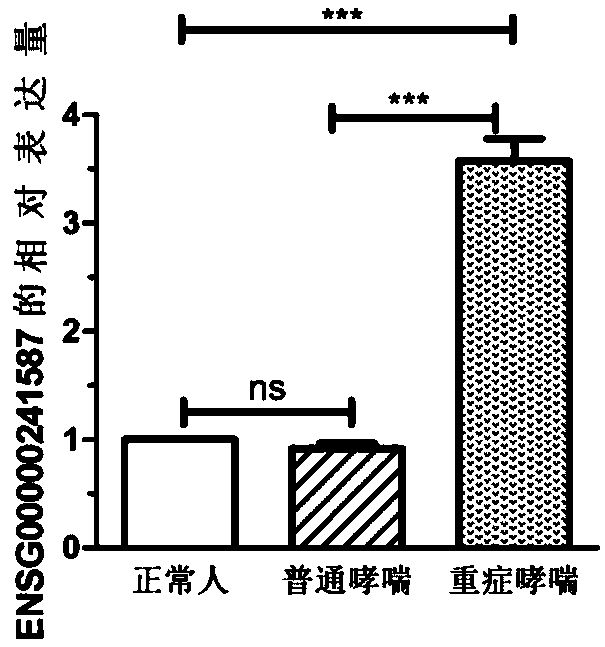
[0078] Example 2 QPCR sequencing to verify the differential expression of the ENSG00000241587 gene
[0079] 1. According to the detection results of high-throughput sequencing, the ENSG00000241587 gene was selected for large-sample QPCR verification. According to the method in Example 1, blood samples were collected from 45 normal asthma patients, 38 severe asthma patients, and 40 normal subjects.
[0080] 2. RNA extraction
[0081] Total RNA was extracted using the RNA extraction kit from Promega, and the specific steps are detailed in the instruction manual.
[0082] 3. Reverse transcription
[0083] The FastQμant cDNA First Strand Synthesis Kit of TIANGEN was used for reverse transcription. For details, please refer to the instruction manual.
[0084] 4. QPCR detection
[0085] 4.1 Primer design
[0086] QPCR amplification primers were designed according to the sequences of the gene encoding ENSG00000241587 and the GAPDH gene, and were synthesized by Shanghai Sangon Bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com