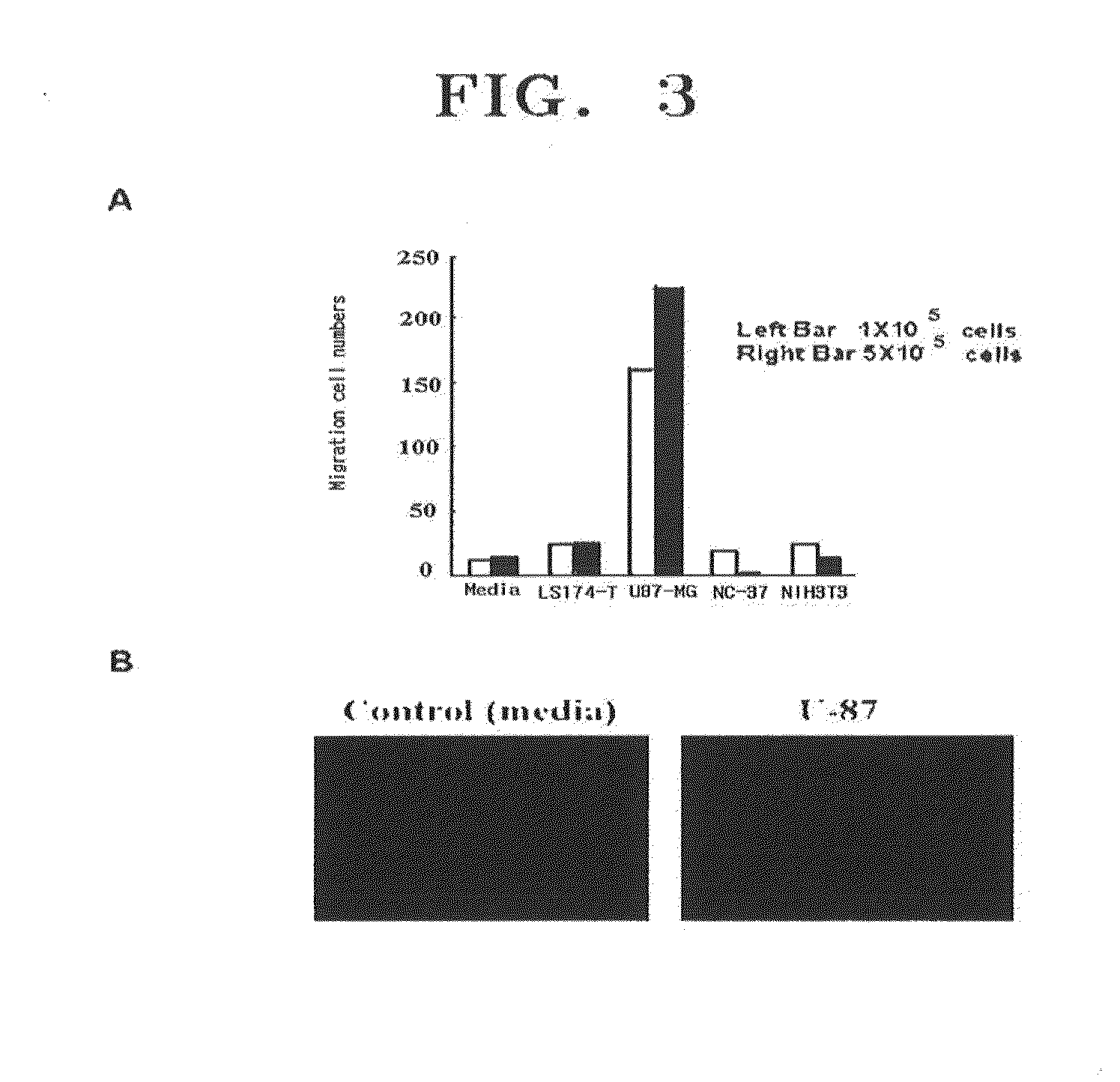
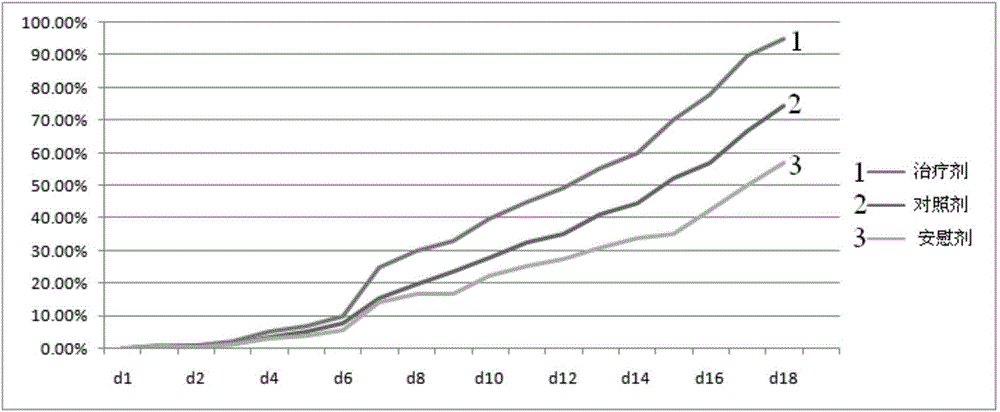
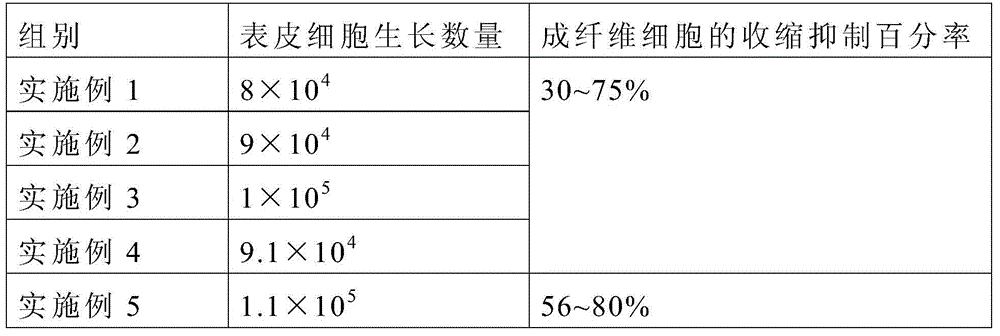
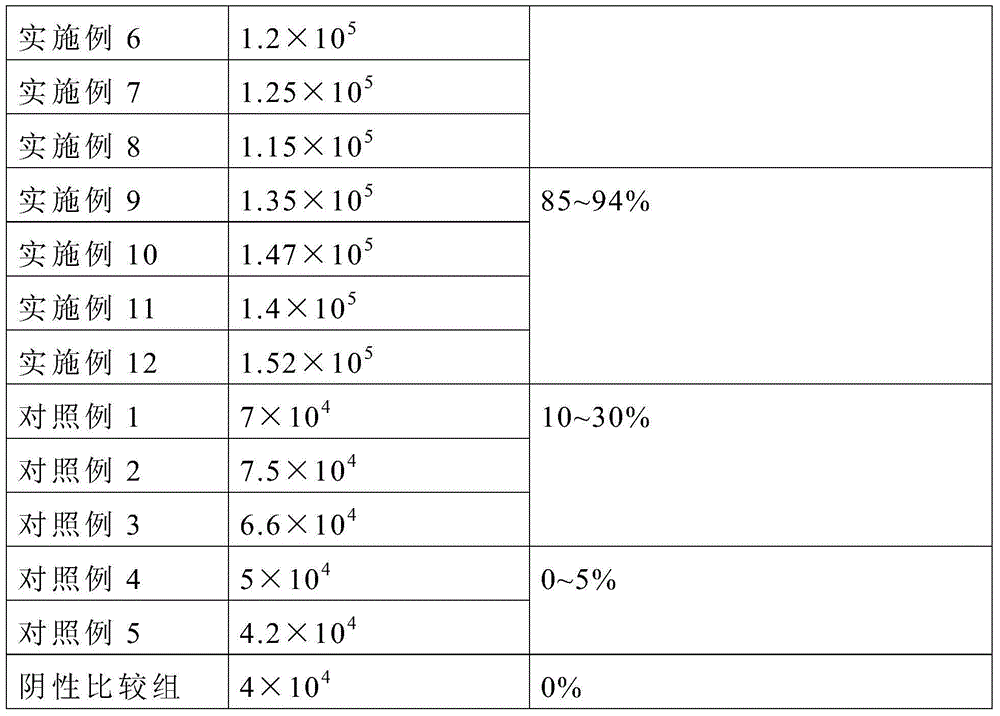
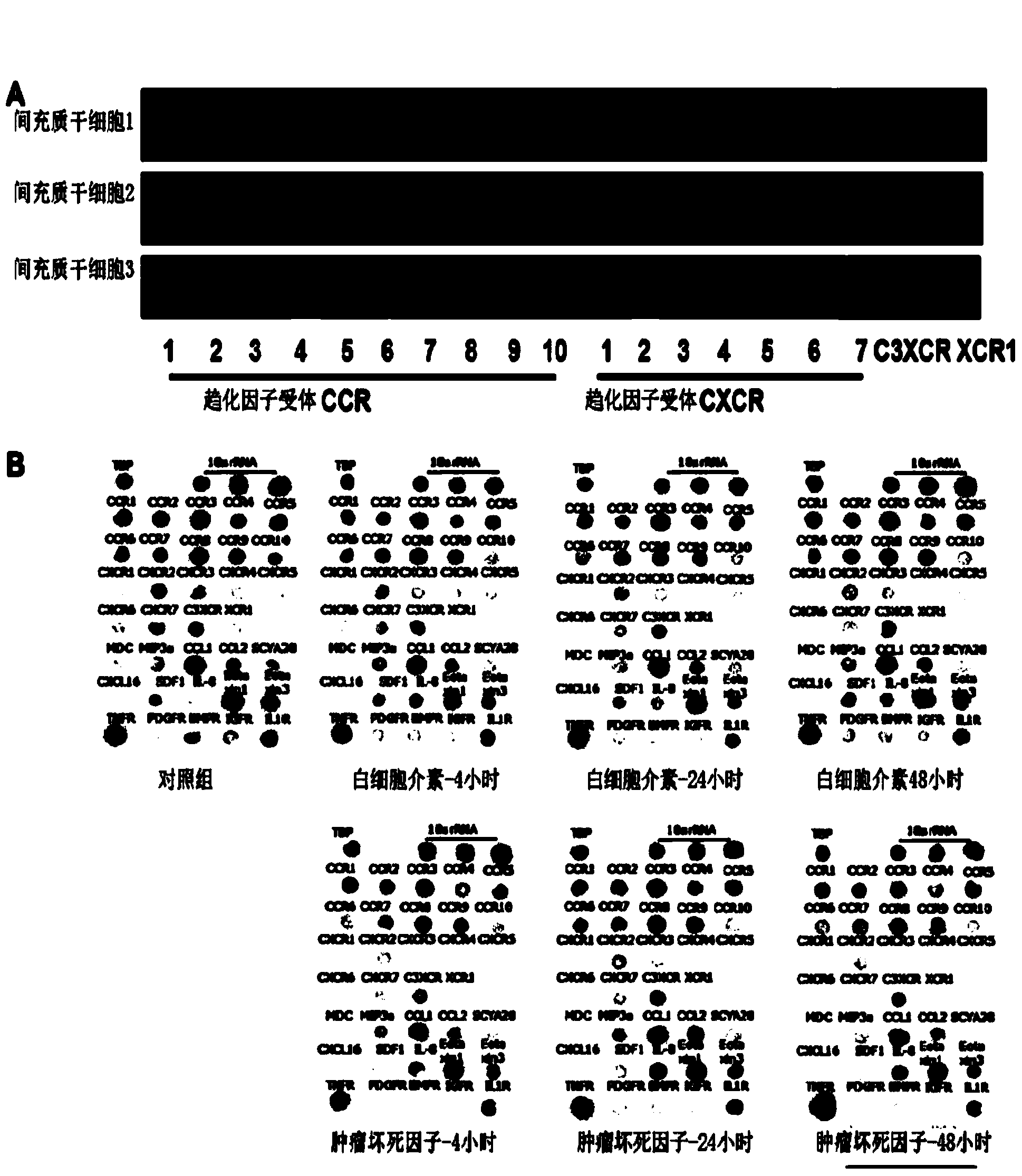
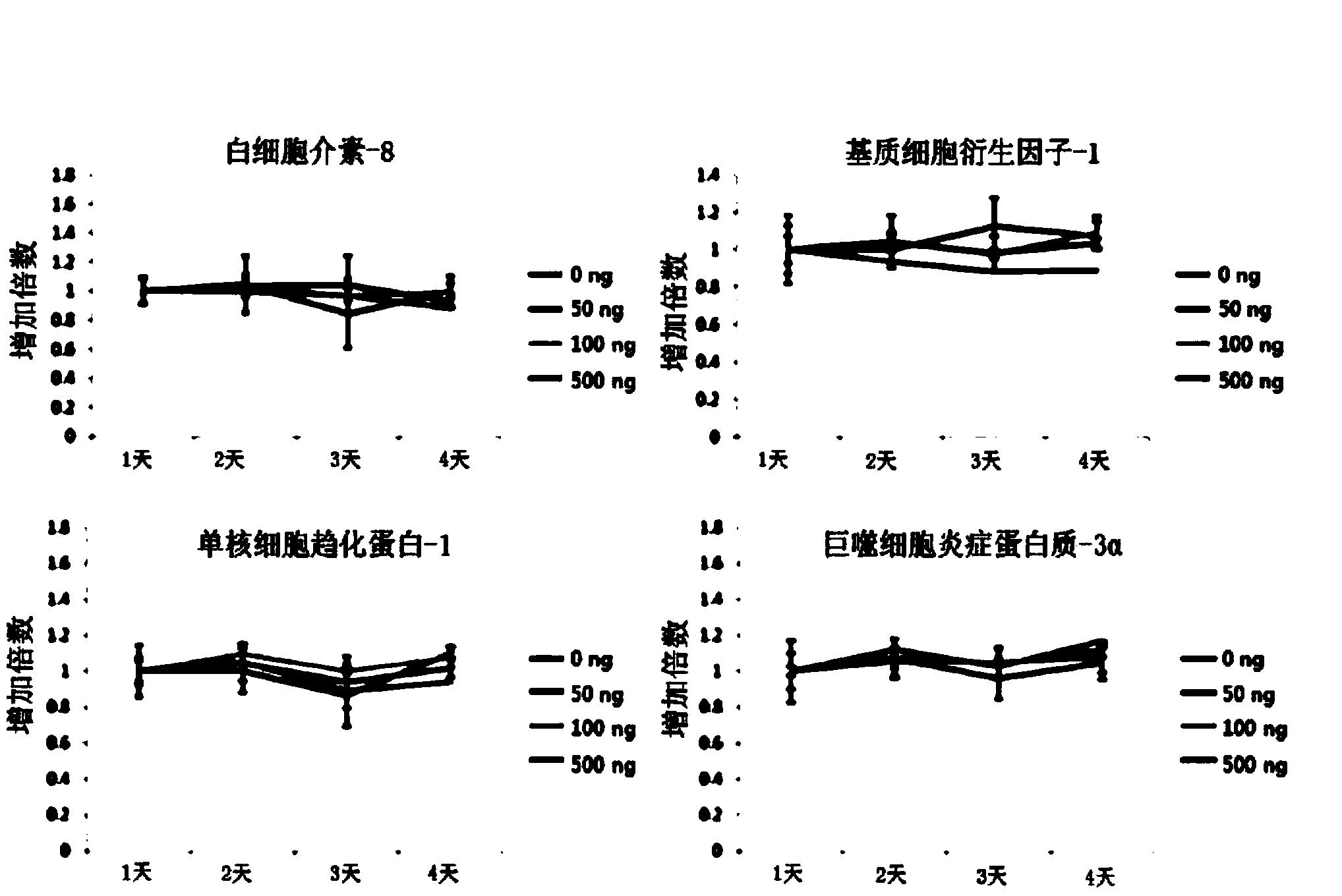
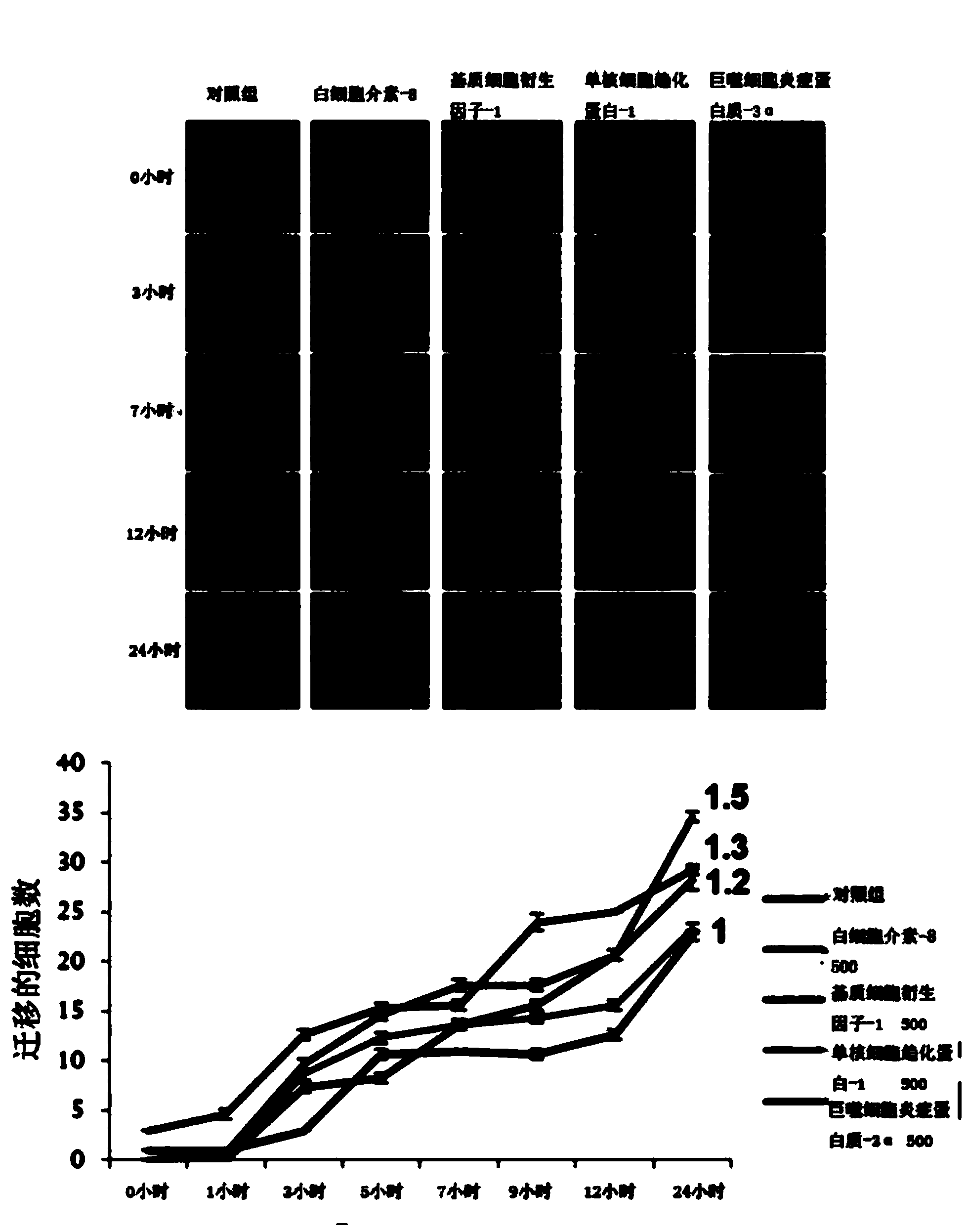
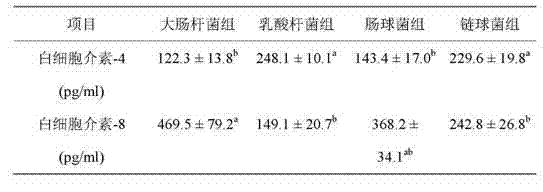
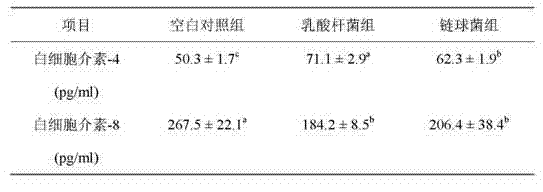
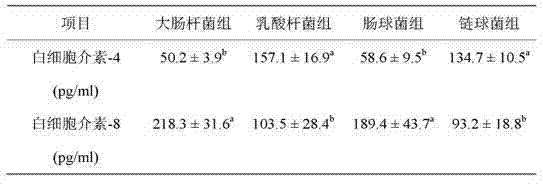
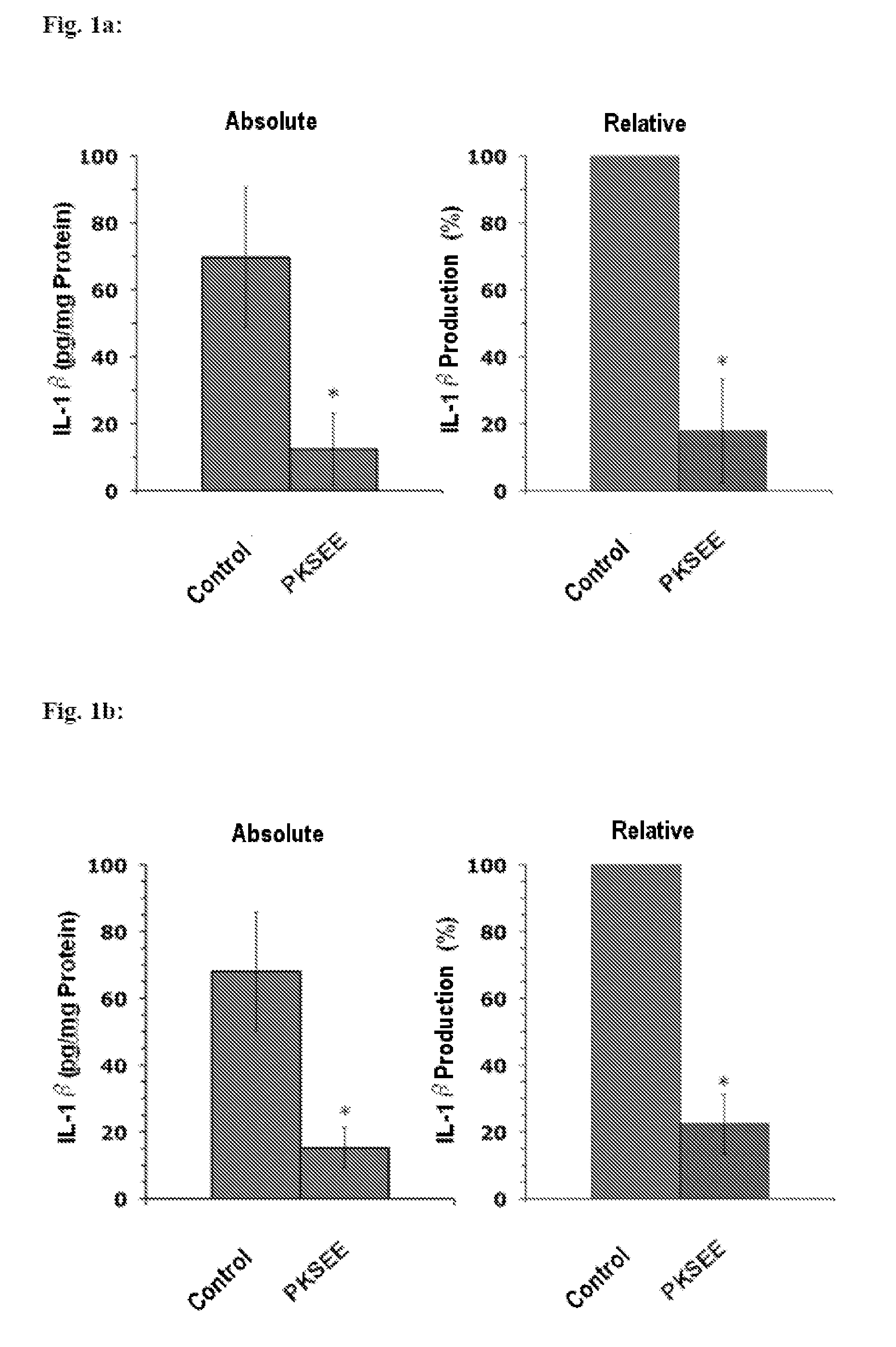
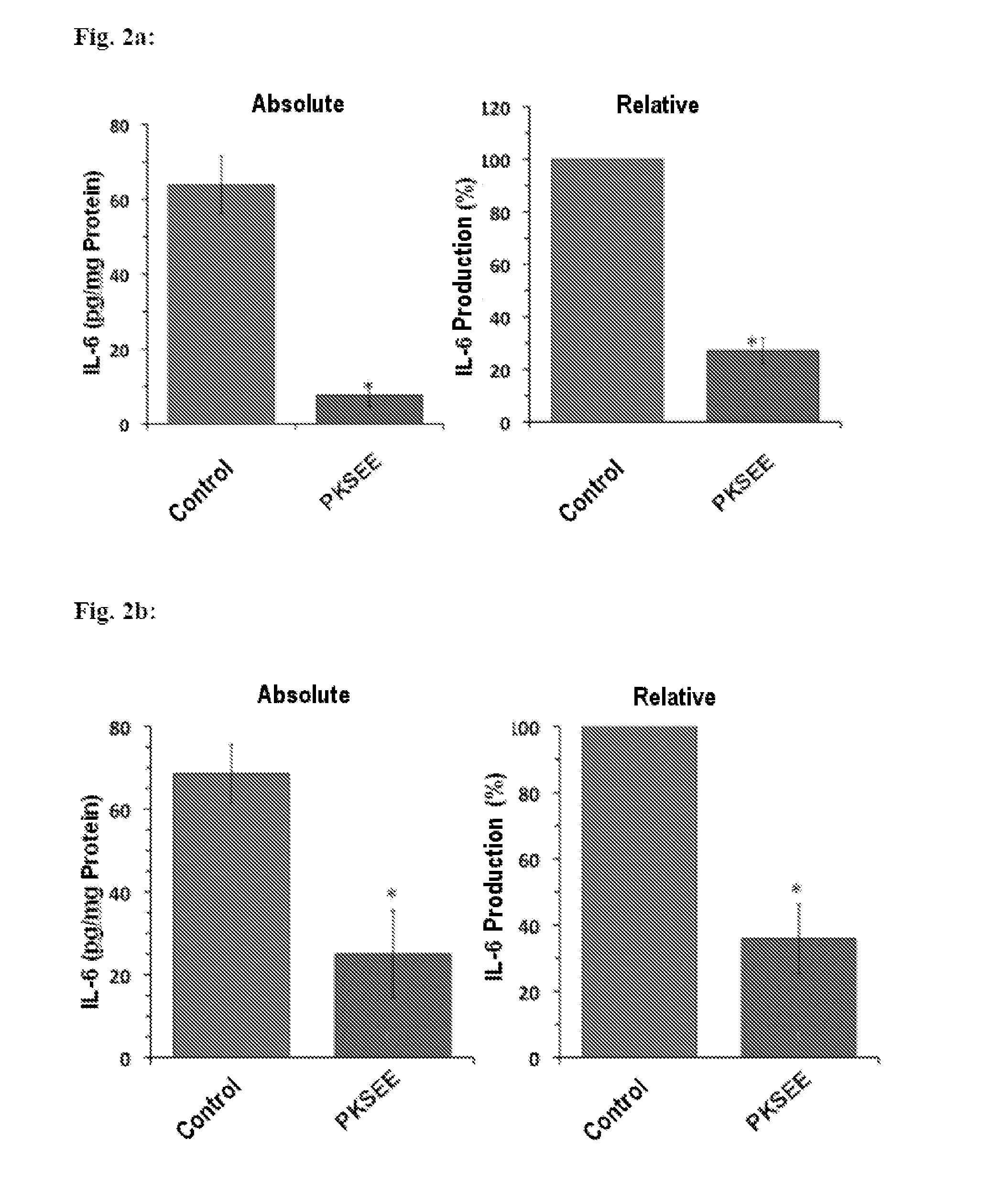
Patents
Literature
88 results about "Interleukin 8" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 8 (IL8 or chemokine (C-X-C motif) ligand 8, CXCL8) is a chemokine produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells and endothelial cells. Endothelial cells store IL-8 in their storage vesicles, the Weibel-Palade bodies. In humans, the interleukin-8 protein is encoded by the CXCL8 gene. IL-8 is initially produced as a precursor peptide of 99 amino acids which then undergoes cleavage to create several active IL-8 isoforms. In culture, a 72 amino acid peptide is the major form secreted by macrophages.
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
Human monoclonal antibodies to interleukin-5
The present invention relates to antibodies and antigen-binding portions thereof that specifically bind to interleukin 5 (IL-5), which is preferably human IL-5. The invention also relates to human anti-IL-5 antibodies, including chimeric, bispecific, derivatized, single chain antibodies or portions of fusion proteins. The invention also relates to isolated heavy and light chain immunoglobulin molecules derived from anti-IL-5 antibodies and nucleic acid molecules encoding such molecules. The present invention also relates to methods of making anti-IL-5 antibodies, pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for diagnosis and treatment. The invention also provides gene therapy methods using nucleic acid molecules encoding the heavy and / or light immunoglobulin molecules that comprise the human anti-IL-5 antibodies. The invention also relates to gene therapy methods and transgenic animals comprising nucleic acid molecules of the present invention.
Owner:MERCK SHARP & DOHME LLC +1
Oral care methods and products
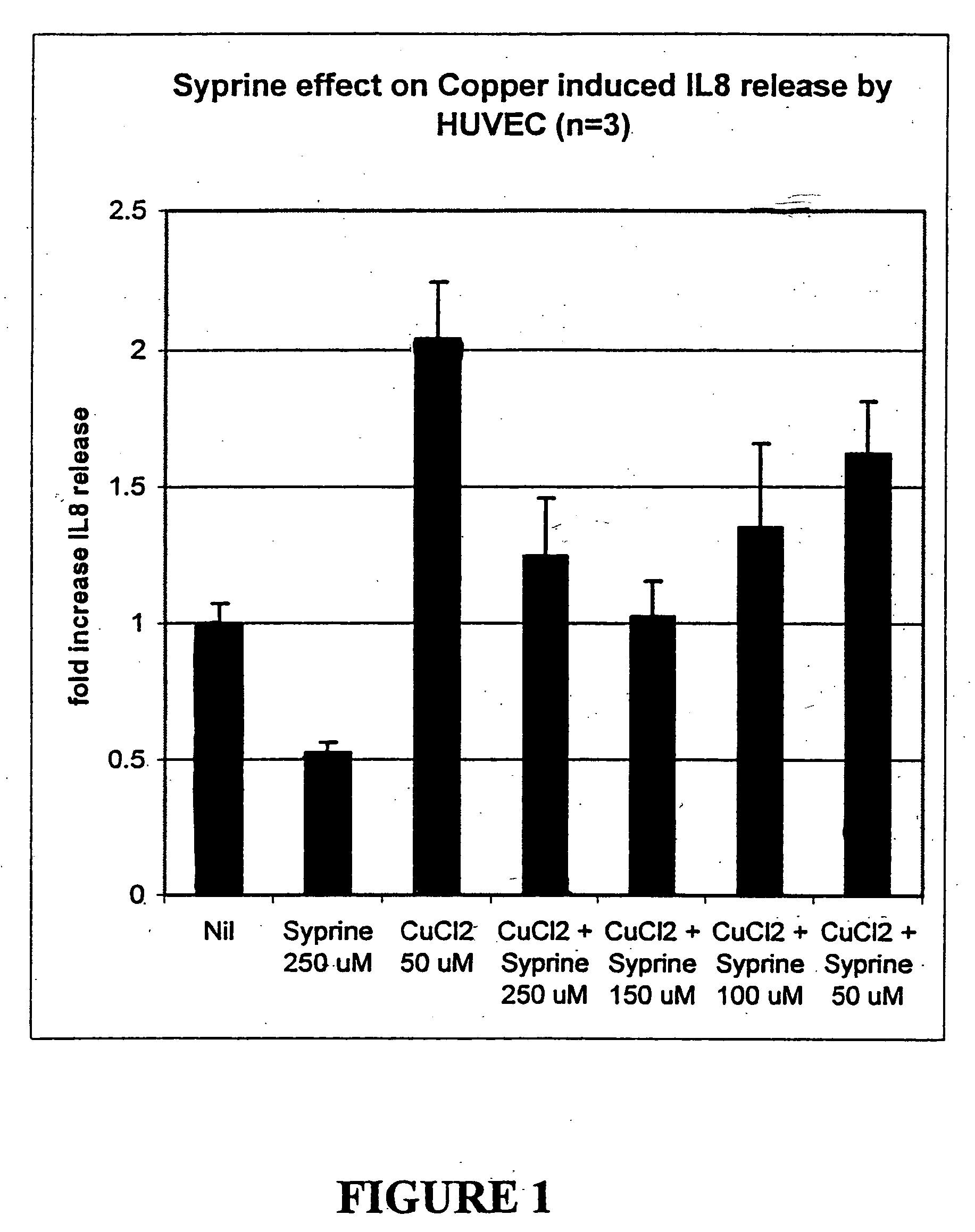
The invention provides methods, oral care products and kits for treating mouth tissues of an animal. In particular, the invention provides methods, oral care products and kits which use or comprise a non-peptide polyamine chelating agent, most preferably trientine, or a physiologically-acceptable salt thereof, which can inhibit the release of pro-inflammatory cytokines, particularly interleukin 8, from cells located in tissues of the mouth and can reduce the damage done by reactive oxygen species (ROS) to such tissues.
Owner:AMPIO PHARMA
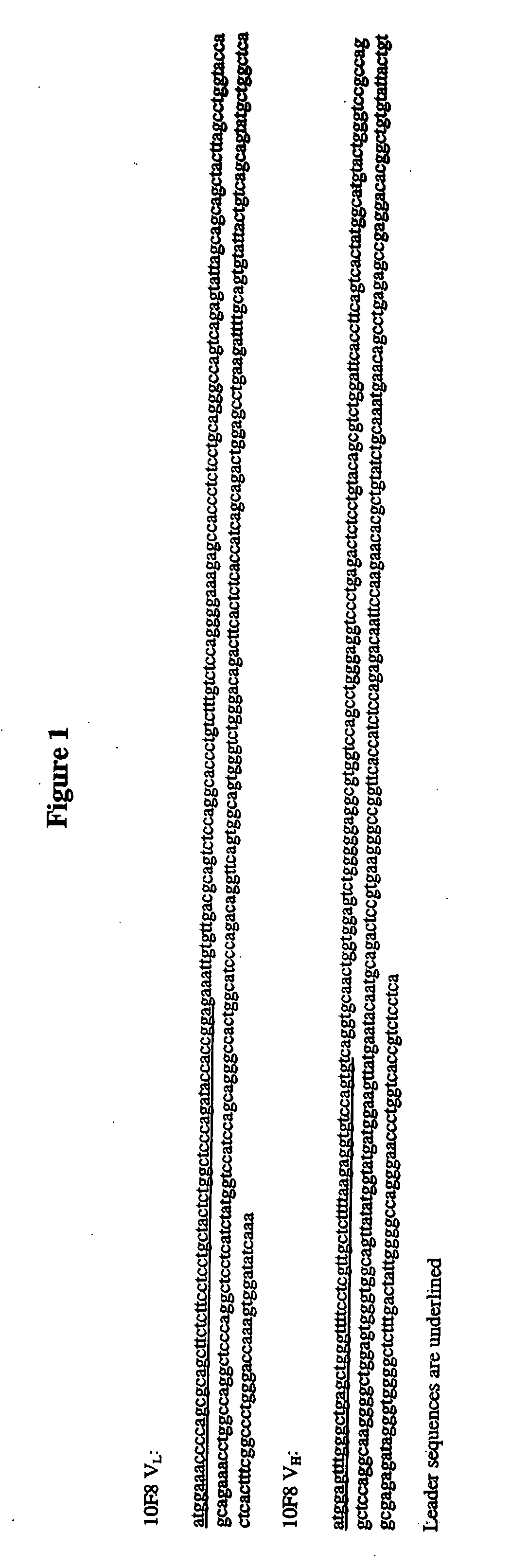
Human monoclonal antibodies against interleukin 8 (IL-8)
ActiveUS7282568B2More therapeutically effectiveLess immunogenicNervous disorderAntipyreticV(D)J recombinationInterleukin 8
Isolated human monoclonal antibodies which bind to IL-8 (e.g., human IL-8) are disclosed. The human antibodies can be produced in a hybridoma, transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals, hybridomas, and transfectomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:CORMORANT PHARMA
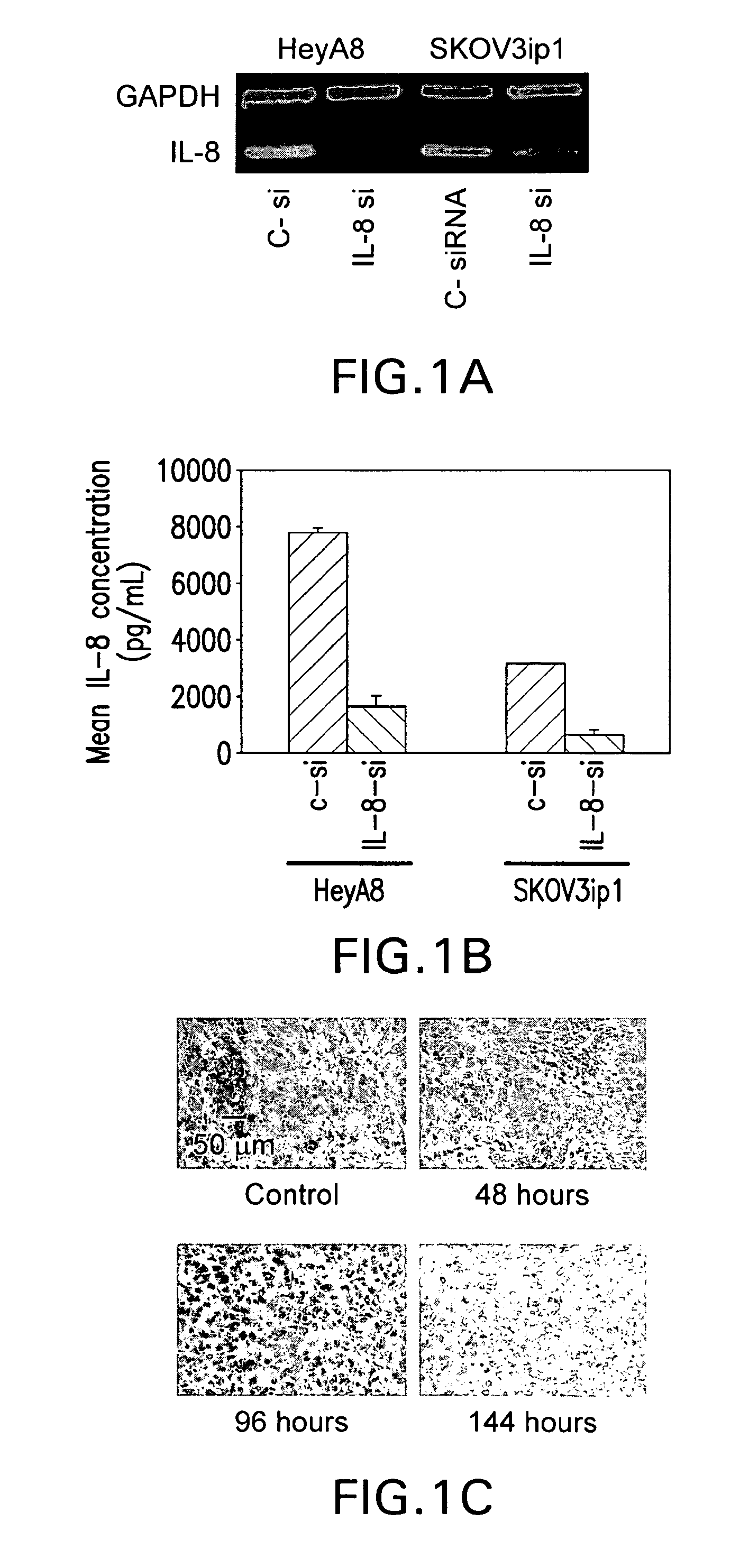
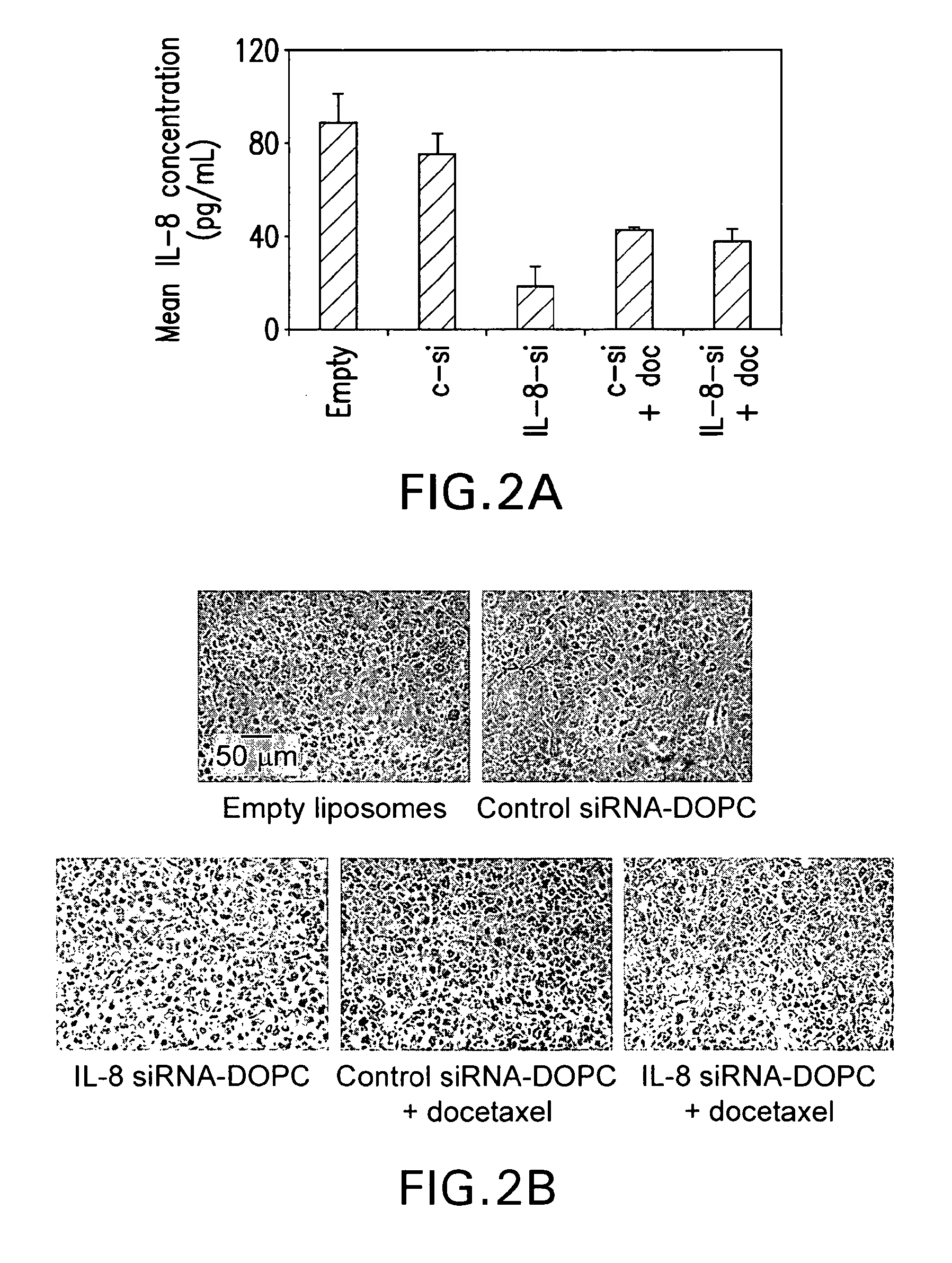
Therapeutic targeting of interleukins using siRNA in neutral liposomes
The present invention relates to the fields of molecular biology and drug delivery. In certain embodiments, the present invention provides compositions that include an siRNA targeted to an interleukin and a neutral lipid, and methods of treating a human subject with cancer involving administering to the subject a pharmaceutically effective amount of an interleukin-8 antagonist or a composition as set forth herein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for treating and monitoring inflammation and redox imbalance cystic fibrosis
InactiveUS20070049641A1Modulating lung inflammationModulating redox imbalance conditionBiocideOrganic active ingredientsWhite blood cellNeutrophil granulocyte
The present invention relates to pharmaceutical kits and methods to treat lung inflammation and redox imbalance in human cystic fibrosis patients using pharmaceutical compositions containing N-acetylcysteine (NAC), pharmaceutically acceptable salts of N-acetylcysteine, or N-acetylcysteine derivatives. In phase I studies, treatment with oral NAC at a dose of from about 1800 mg / day to about 3000 mg / day for a period of 4 weeks produced significant positive effects, namely, it decreased absolute numbers of white blood cells and neutrophils in the sputum and produced concomitant decreases in sputum neutrophil elastase specific activity and sputum interleukin-8 levels, suggesting an amelioration of lung inflammation in the patients. These effects were associated with an increased total GSH level in whole blood as well increased staining for reduced GSH in blood neutrophils, both of which reflect an amelioration of the redox imbalance in the patients. In ongoing phase II studies, oral NAC at a dose of about 2700 mg / day administered in double-blind manner for 12 weeks showed excellent safety and significantly decreased white blood cells in sputum as compared to placebo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
High affinity fully human monoclonal antibodies to interleukin-8 and epitopes for such antibodies
InactiveUS20090130110A1Organic active ingredientsAntipyreticEpitopeComplementarity determining region
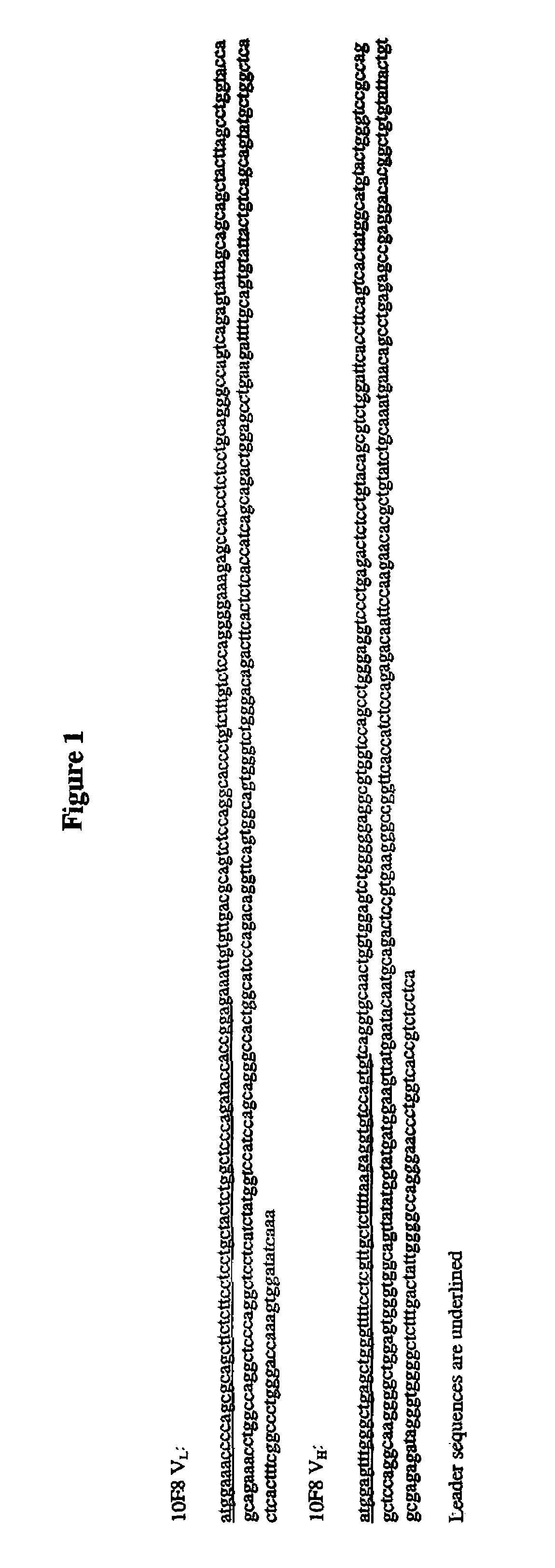
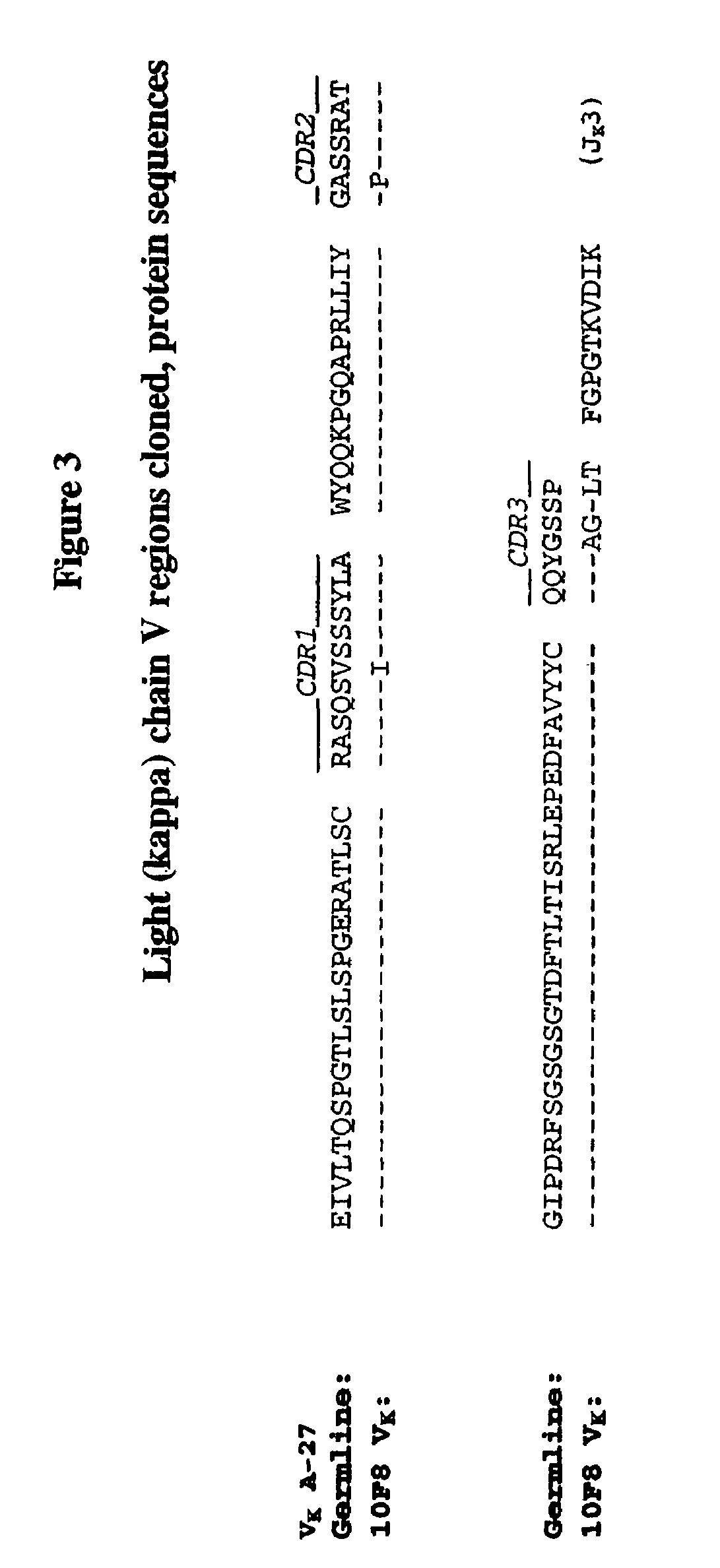
The present embodiments are related to high-affinity antibodies directed to IL-8, methods of making and characterizing such antibodies and uses of such antibodies. Isolated polynucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions (FR's) and / or complementarity determining regions (CDR's), are provided.
Owner:AMGEN FREMONT INC
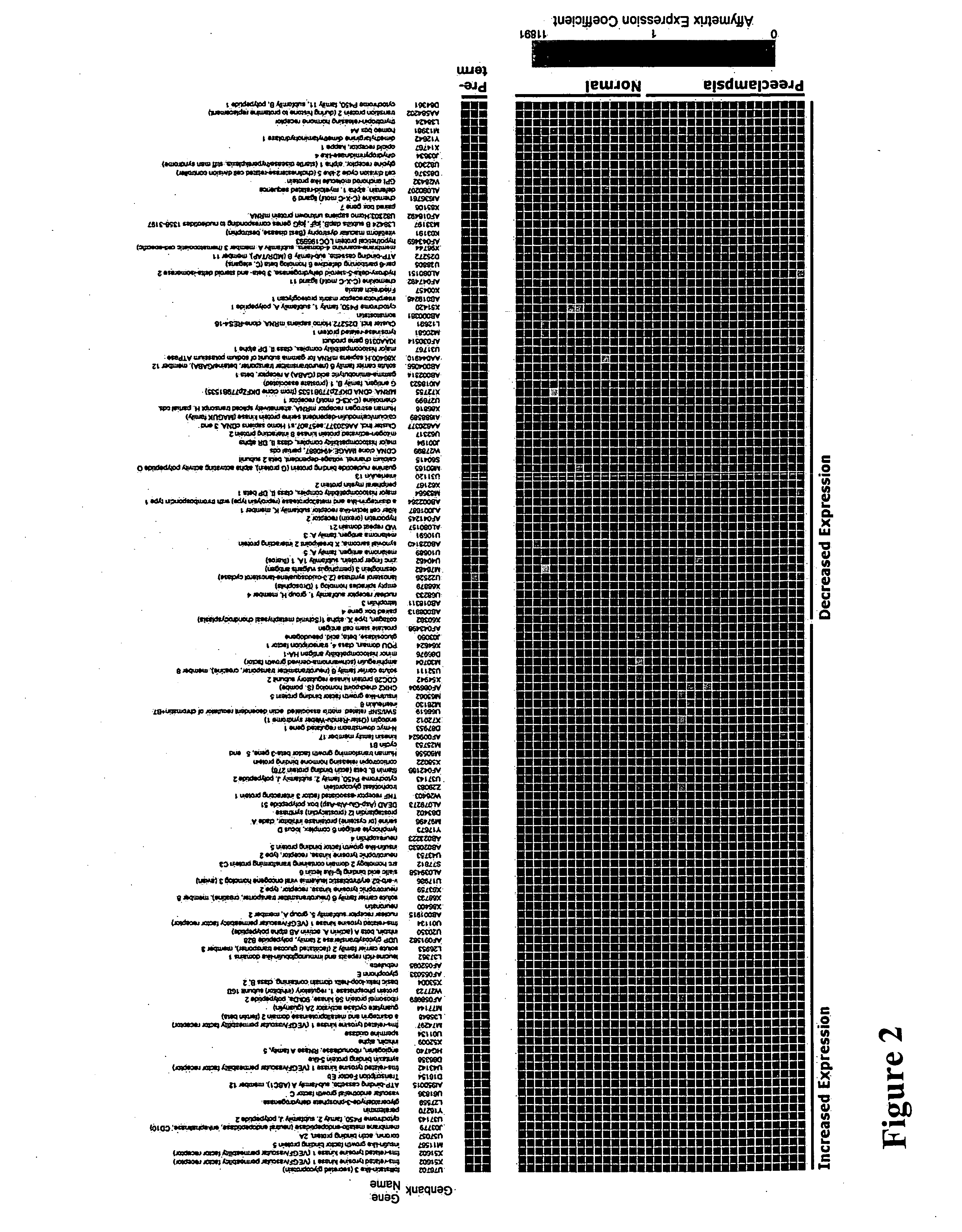
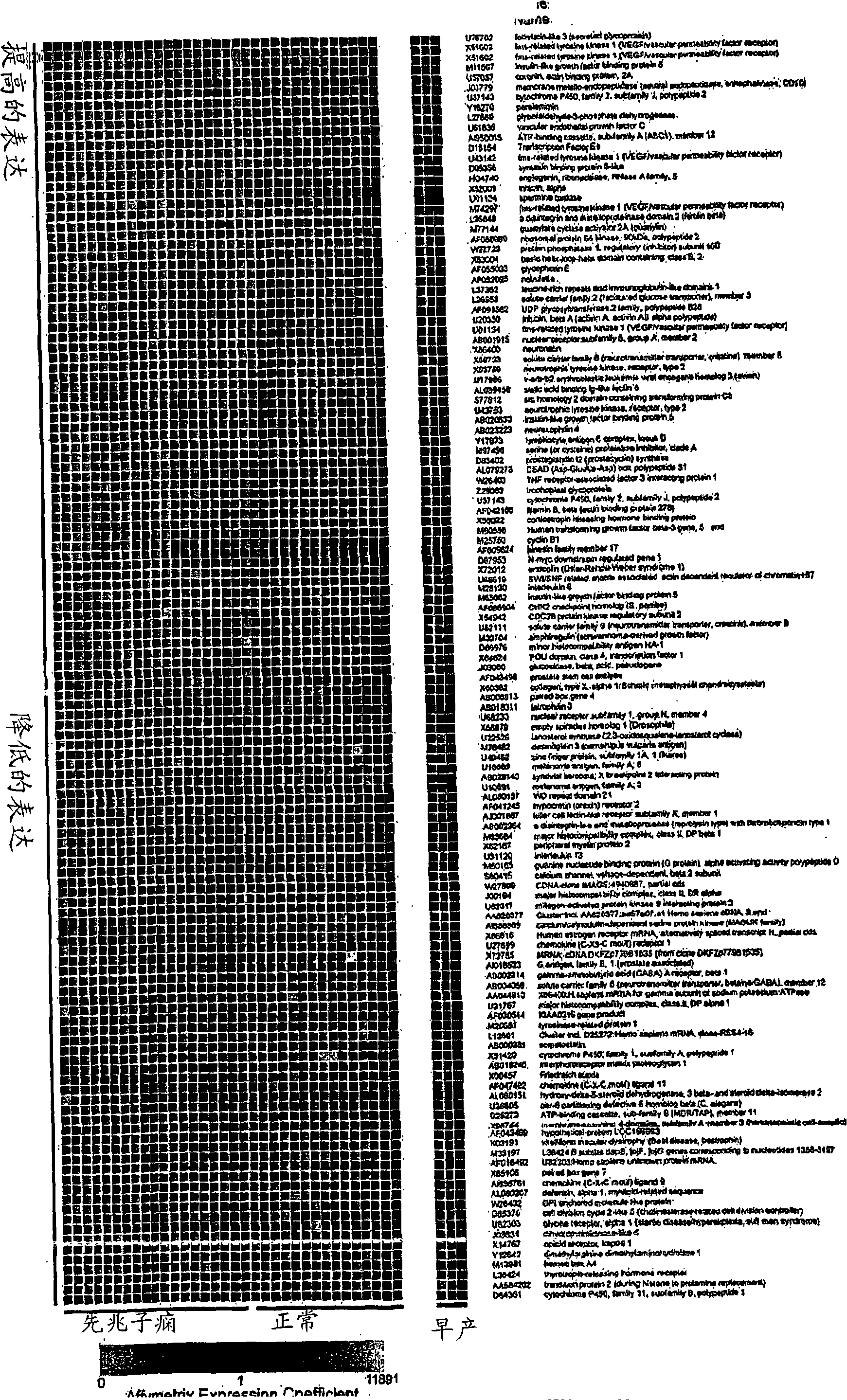
Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
ActiveUS20060166277A1Diagnosing and effectively treatingSave maternalMicrobiological testing/measurementDisease diagnosisPregnancyUdp glycosyltransferase
Disclosed herein are methods for diagnosing or treating pregnancy related hypertensive disorders that include the use of a polypeptide or a nucleic acid encoding a polypeptide selected from the following: follistatin related protein, interleukin 8, inhibin A, VEGF-C, angiogenin, beta fertilin, hypothetical protein, leukocyte associated Ig-like receptor secreted protein, erythroid differentiation protein, adipogenesis inhibitory factor, corticotropin releasing factor binding protein, alpha-1 anti-chymotrypsin, insulin-like growth factor binding protein-5, CD33L, cytokine receptor like factor 1, platelet derived endothelial growth factor, lysyl hydroxylase isoform 2, stanniocalcin precursor, secreted frizzled related protein, galectin-3, alpha defensin, ADAM-TS3, cholecystokinin precursor, interferon stimulated T-cell alpha chemoattractant precursor, azurocidin, sperminine oxidase, UDP glycosyltransferase 2 family polypeptide B28, neurotrophic tyrosine kinase receptor 2, neutral endopeptidase, CDC28 protein kinase regulatory subunit 2, beta glucosidase, lanosterol synthase, calcium / calmodulin-dependent serine protein kinase, estrogen receptor-alternatively spliced transcript H, chemokine (CX3C motif) receptor 1, tyrosinase-related protein 1, hydoxy-delta-5-steroid dehyrogenase, dihydropyramidinase-like-4, and cytochrome P450-family 11.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
High affinity fully human monoclonal antibodies to interleukin-8
The present embodiments are related to high-affinity antibodies directed to IL-8, methods of making and characterizing such antibodies and uses of such antibodies. Isolated polynucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions (FR's) and / or complementarity determining regions (CDR's), are provided.
Owner:AMGEN FREMONT INC
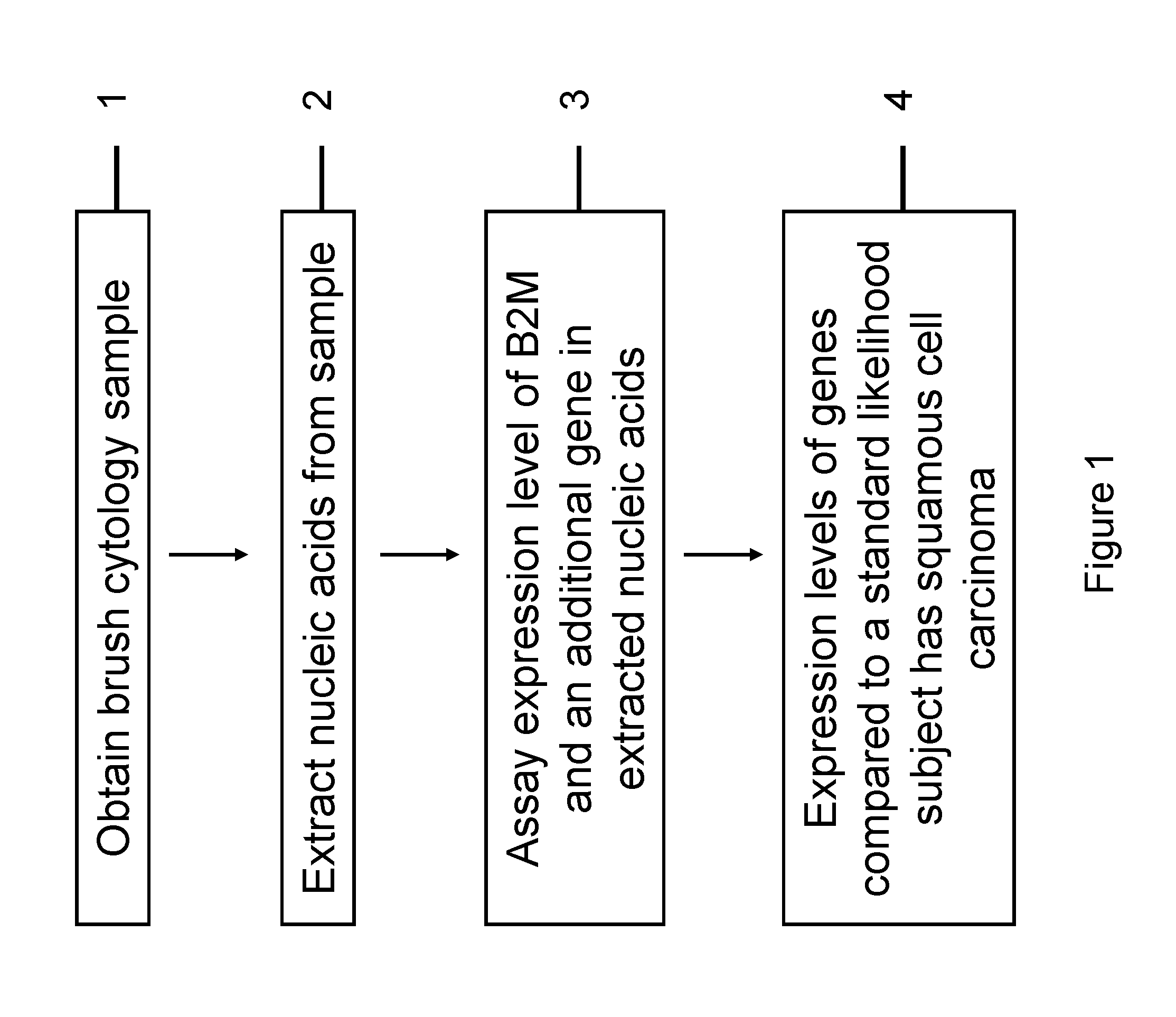
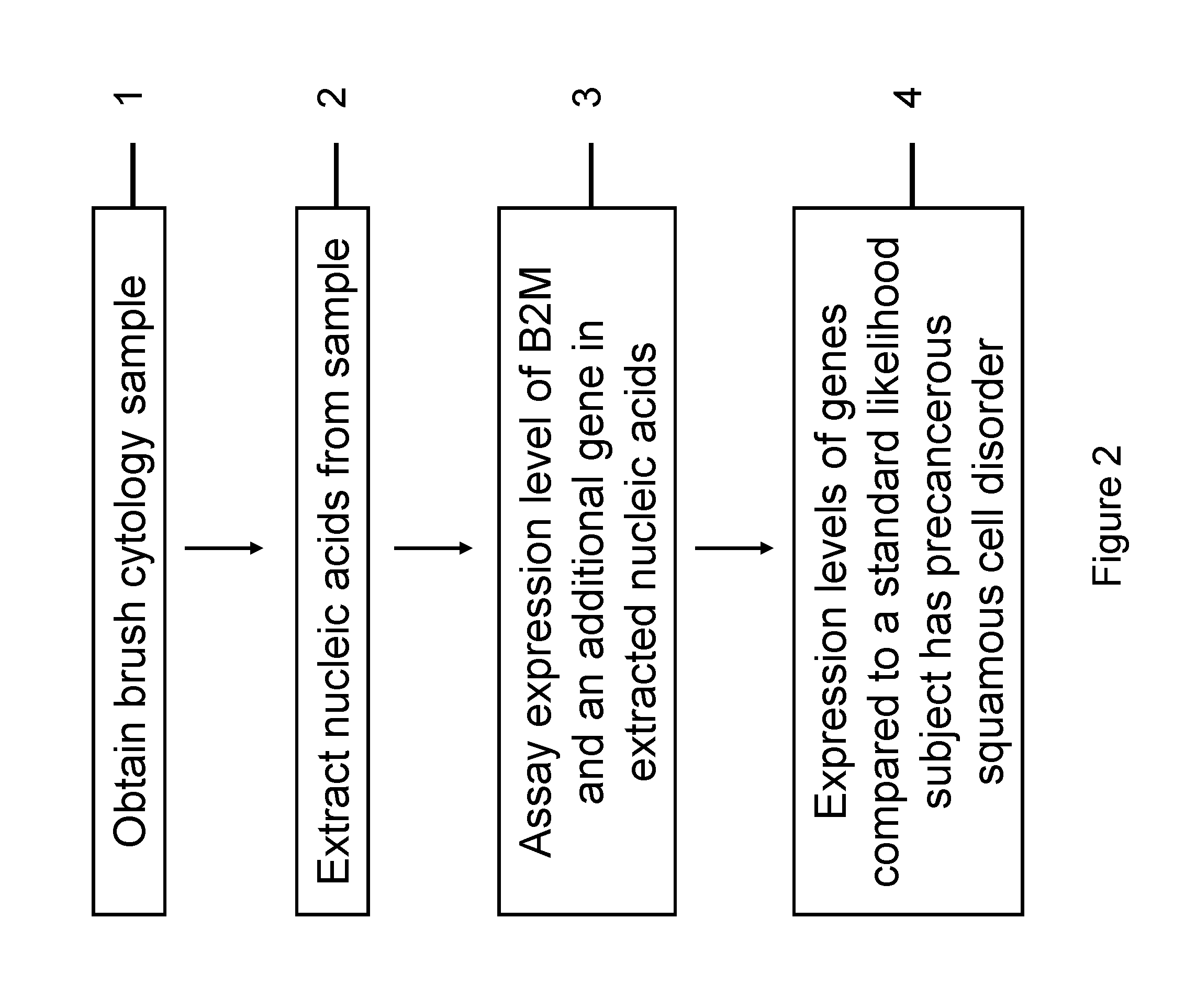
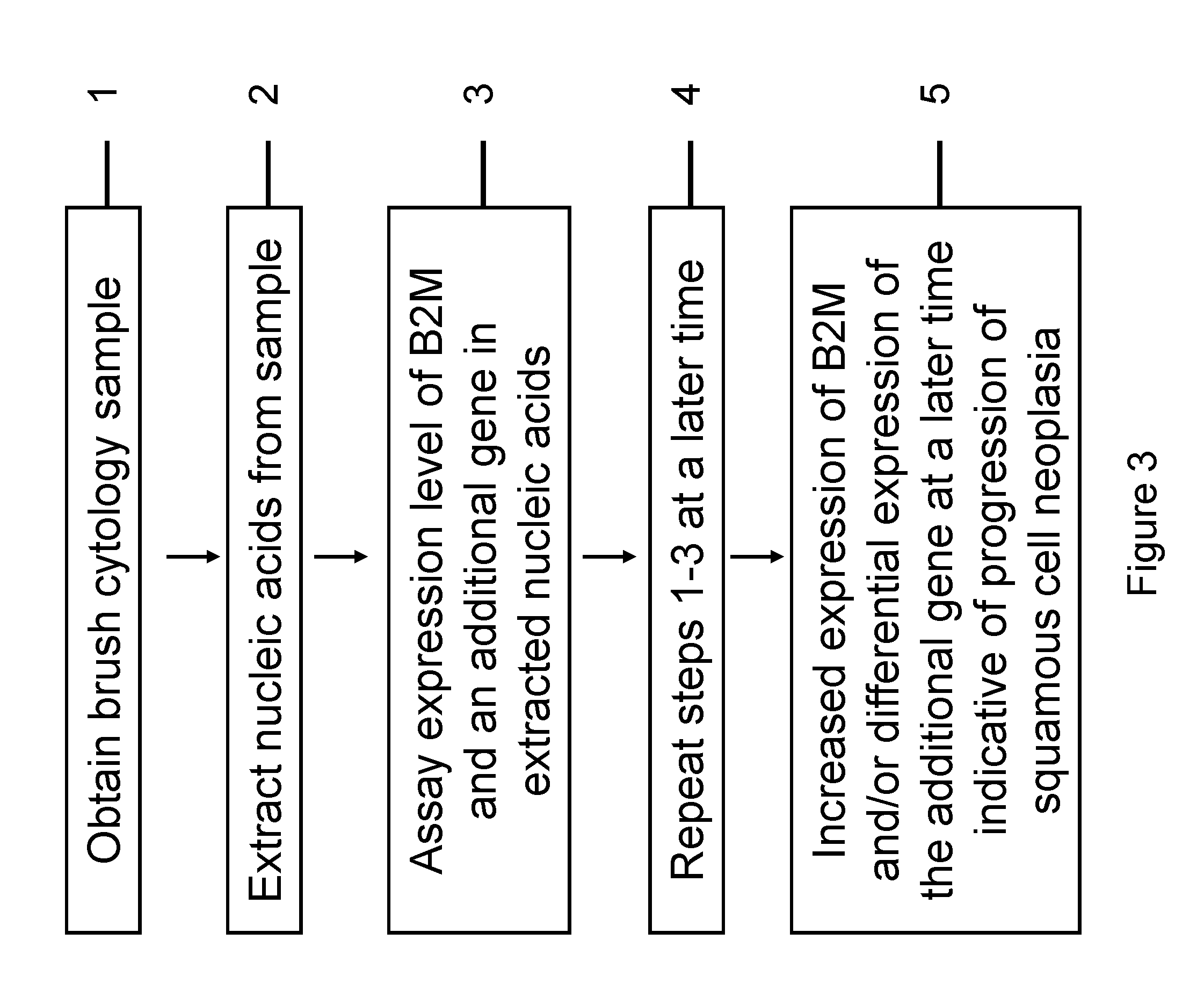
RNA from cytology samples to diagnose disease
InactiveUS20120231468A1Monitor progressMicrobiological testing/measurementSquamous CarcinomasWhite blood cell
The invention relates to methods and kits for detecting the likelihood that a subject has cancer, e.g., squamous cell carcinoma, by assaying the expression levels of tumor associated genes. More specifically, the expression levels of nucleic acids or proteins can be assayed in the tumor associated genes, e.g., over-expression of beta-2 microgobulin (B2M), keratin 17 (KRT17), interleukin 8 (IL8), or annexin A2 (ANXA2), and under-expression of cytochrome p450 1B1 (CYP1B1) or laminin gamma-2 (LAMC2) can be indicative of the likelihood a subject has squamous cell carcinoma or a precancerous squamous cell disorder. The expression levels compared to standards can be indicative of the likelihood a subject has squamous cell carcinoma. The expression levels of B2M, CYP1B1, KRT17, IL8, ANXA2, or LAMC2 can also be repeatedly assayed to monitor the progression of a squamous cell neoplasia.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Human monoclonal antibodies against interleukin 8 (IL-8)
InactiveUS20080118517A1More therapeutically effectiveLess immunogenicNervous disorderAntipyreticV(D)J recombinationInterleukin 8
Isolated human monoclonal antibodies which bind to IL-8 (e.g., human IL-8) are disclosed. The human antibodies can be produced in a hybridoma, transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals, hybridomas, and transfectomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:CORMORANT PHARMA
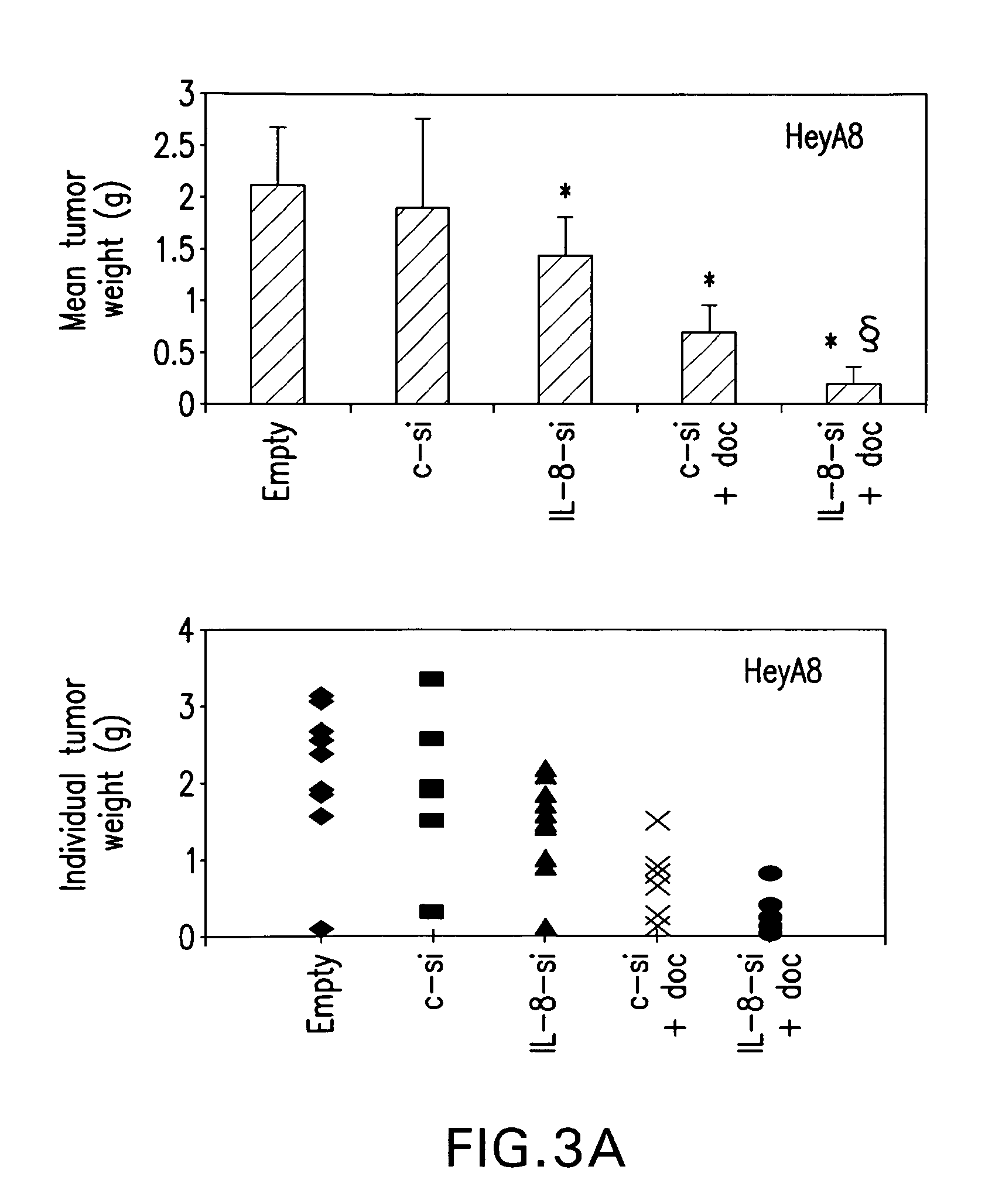
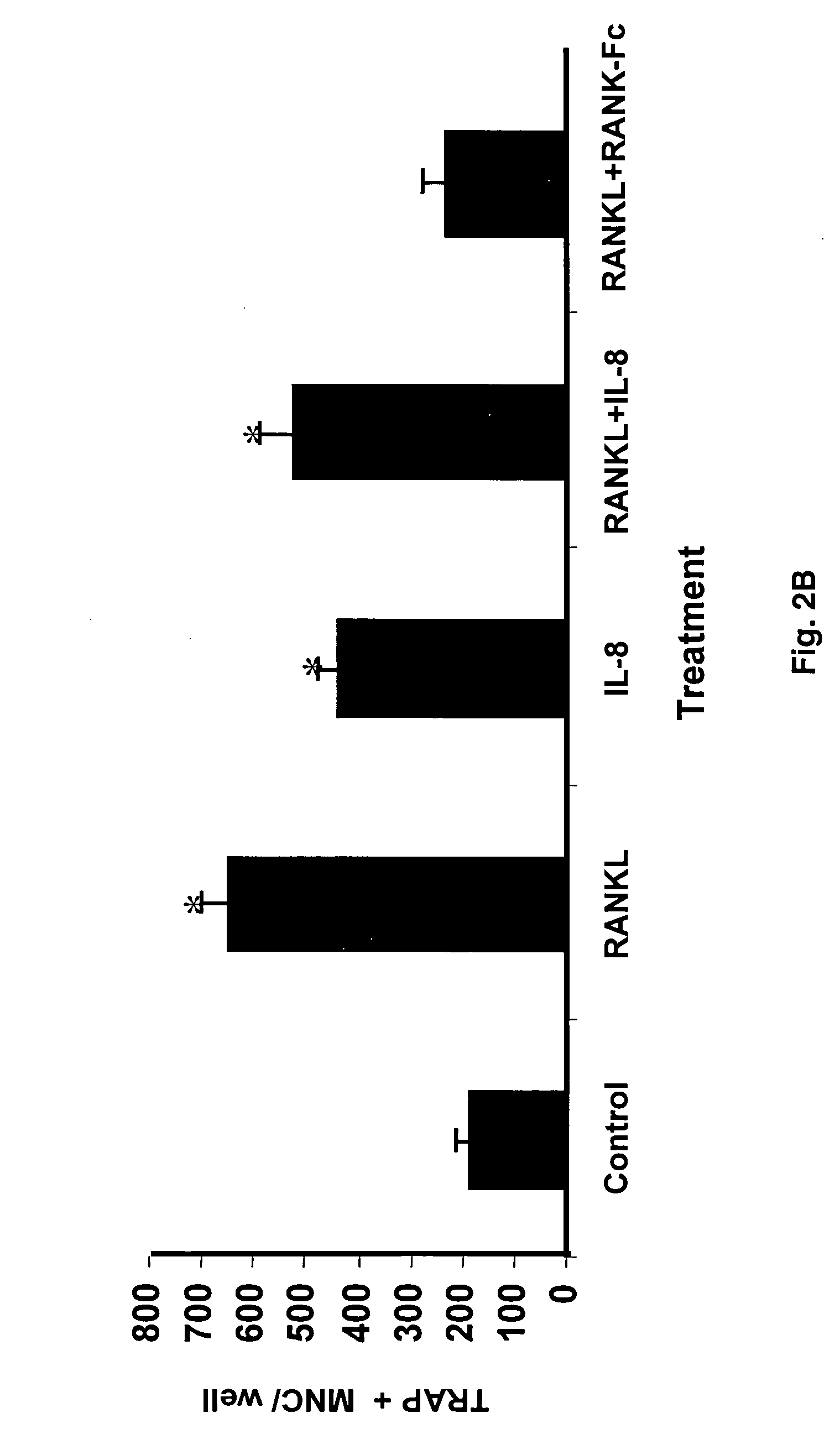
Anti-interleukin 8 therapy for tumor osteolysis
InactiveUS20050142136A1Stimulate osteoclastic bone resorptionRegulate expressionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsAbnormal tissue growthCancer cell
The present invention reports stimulatory effects of interleukin 8 (IL-8) on human osteoclast formation and bone resorption, indicating IL-8 as a potent activator of bone destruction common in metastatic bone diseases. Tumor growth and osteolysis were inhibited by anti-IL-8 antibody or antisense IL-8. Additionally, IL-8 was able to confer an osteolytic phenotype on non-osteolytic cancer cells. These results identify tumor-induced osteolysis and bone resorption as potential targets of anti-IL-8 therapy.
Owner:ARKANSAS FOR MEDICAL SCI UNIV OF THE
Methods and Kits for Predicting Treatment Response in Type II Diabetes Mellitus Patients
InactiveUS20100273661A1Improve patient 's clinical response rateHigh response rateData processing applicationsMicrobiological testing/measurementSupport vector machineCITRATE ESTER
A method for predicting treatment response of a type II diabetes patient to rosiglitazone is provided. The method involves at least one sample from a patient having type II diabetes and analyzing biomarkers predictive of a patient who will respond to treatment with rosiglitazone. The biomarkers include, at least, interleukin-8, histidine, citrate. These biomarkers are identified in at least one classification analyses selected from the group consisting of a majority-vote based classifier and support-vector machine (SVM) classifier. Also provided is a method for predicting treatment response of a type II diabetes patient to glyburide at 8 weeks post-initiation of therapy. The method involves obtaining a sample from a type II diabetes patient who has been treated with glyburide for about 4 weeks and analyzing biomarkers predictive of a patient who will respond to treatment with glyburide at 8 weeks. The biomarkers useful in this method include, at least, sphingomyelin 23:1 and L-phenylalanine. Also provided are kits useful for the methods of the invention.
Owner:SMITHKLINE BECKMAN CORP +1
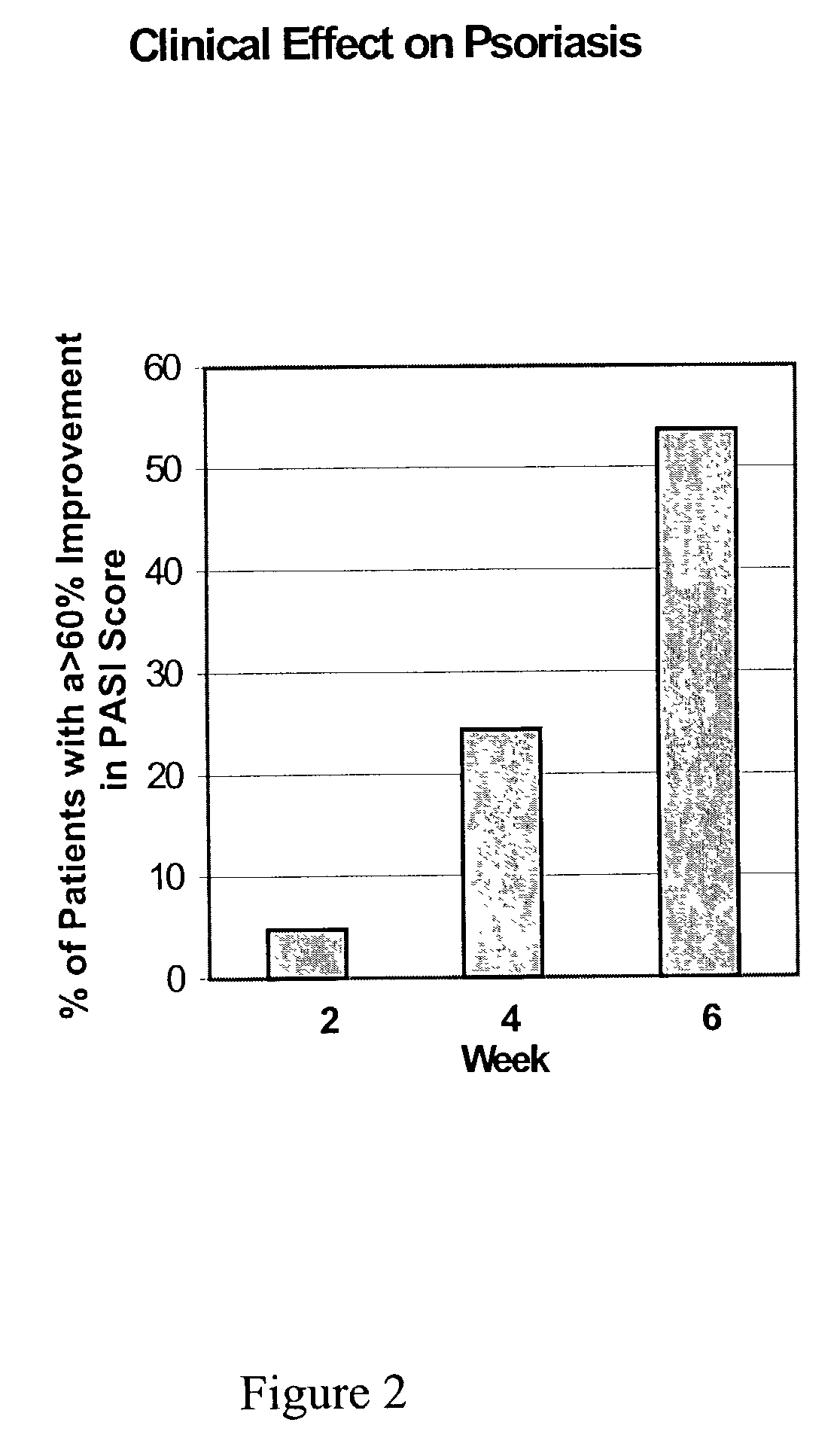
Topical treatment of psoriasis using neutralizing antibodies to interleukin-8
InactiveUS7147854B2Good treatment effectPeptide/protein ingredientsSnake antigen ingredientsAntibody fragmentsInterleukin 8
The present invention provides a topical preparation for treating patients with skin inflammatory disease. The topical preparation contains an antibody to interleukin-8 (IL-8) and a pharmaceutically acceptable carrier. The antibody can be a monoclonal antibody, a polyclonal antibody, an antibody fragment, or a combination thereof. The monoclonal antibody is a murine anti-human IL-8 monoclonal antibody produced in a hybridoma. The polyclonal antibody is a chicken anti-human polyclonal antibody, which is prepared by immunizing a chicken with human IL-8, collecting eggs from the immunized chicken, and purifying the IgY from the eggs. The topical preparation is effective in topically treating inflammatory skin diseases. The present invention also provides a method for treating patients with skin inflammatory disease, which is by topically applying an effective amount of the topical preparation onto patients with skin inflammatory disease.
Owner:YES BIOTECH LAB
Nucleic acids and polypeptides useful for diagnosing and treating complications of pregnancy
InactiveCN101299962AMicrobiological testing/measurementDisease diagnosisAlpha defensinInsulin-like growth factor-binding protein
Disclosed herein are methods for diagnosing or treating pregnancy related hypertensive disorders that include the use of a polypeptide or a nucleic acid encoding a polypeptide selected from the following: follistatin related protein, interleukin 8, inhibin A, VEGF-C, angiogenin, beta fertilin, hypothetical protein, leukocyte associated Ig-like receptor secreted protein, erythroid differentiation protein, adipogenesis inhibitory factor, corticotropin releasing factor binding protein, alpha-1- anti-chymotrypsin, insulin-like growth factor binding protein-5, CD33L, cytokine receptor like factor 1, platelet derived endothelial growth factor, lysyl hydroxylase isoform 2, stanniocalcin precursor, secreted frizzled related protein, galectin-3, alpha defensin, ADAM-TS3, cholecystokinin precursor, interferon stimulated T-cell alpha chemoattractant precursor, azurocidin, sperminine oxidase, UDP glycosyltransferase 2 family polypeptide B28, neurotrophic tyrosine kinase receptor 2, neutral endopeptidase, CDC28 protein kinase regulatory subunit 2, beta glucosidase, lanosterol synthase, calcium / calmodulin-dependent serine protein kinase, estrogen receptor-alternatively spliced transcript H, chemokine (CX3C motif) receptor 1, tyrosinase-related protein 1, hydoxy-delta-5-steroid dehyrogenase, dihydropyramidinase-like-4, and cytochrome P450-family 11.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
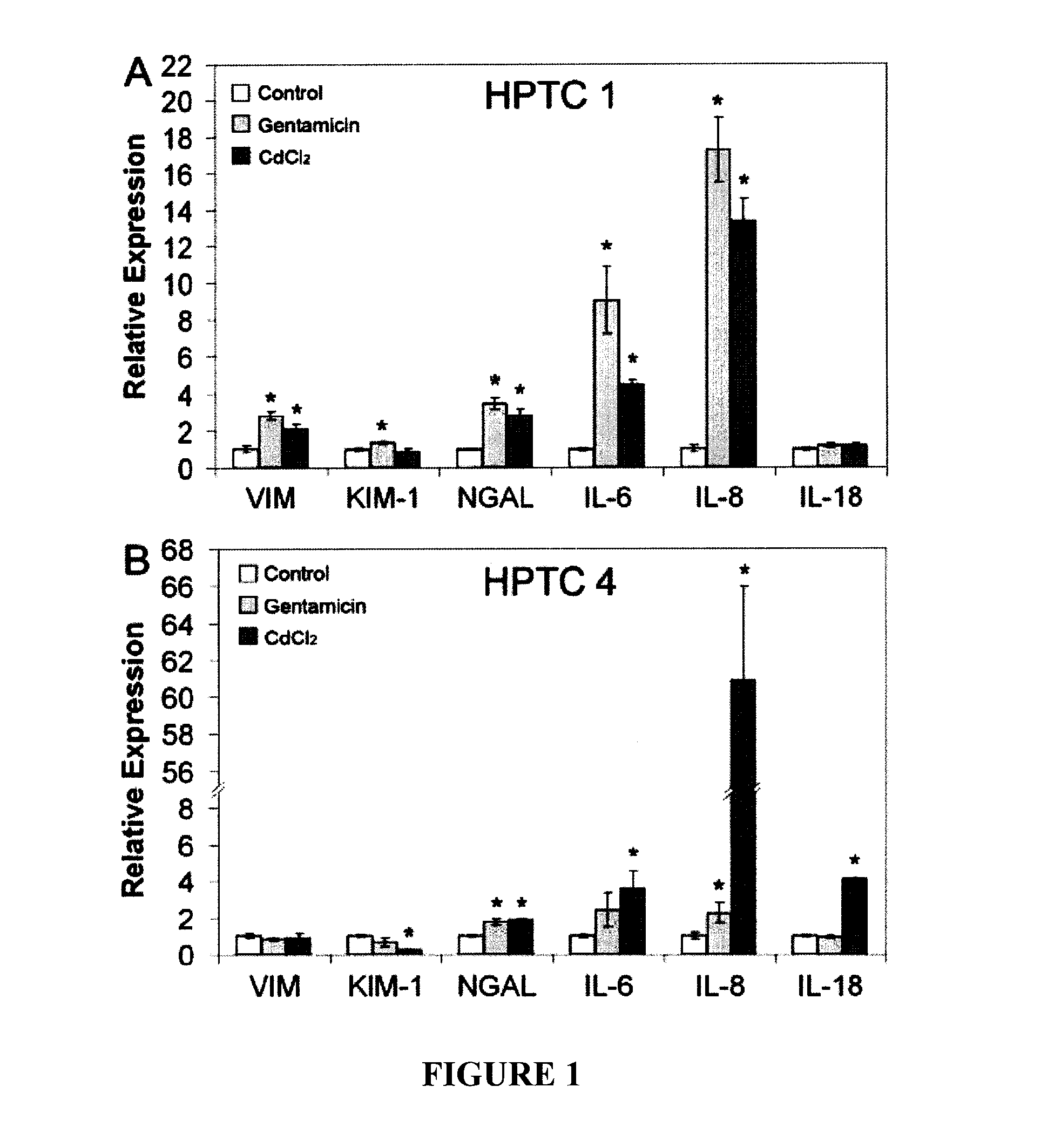
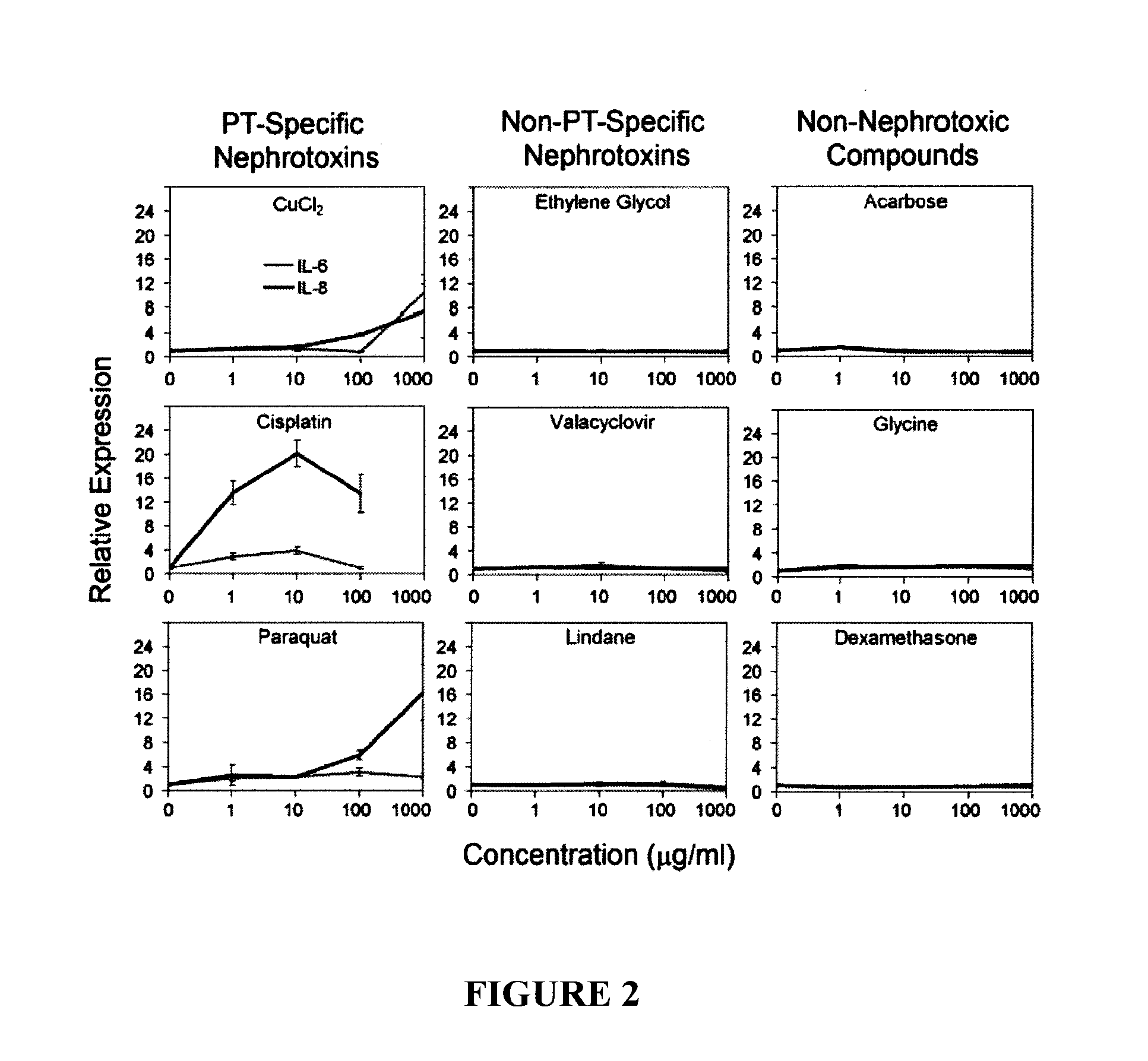
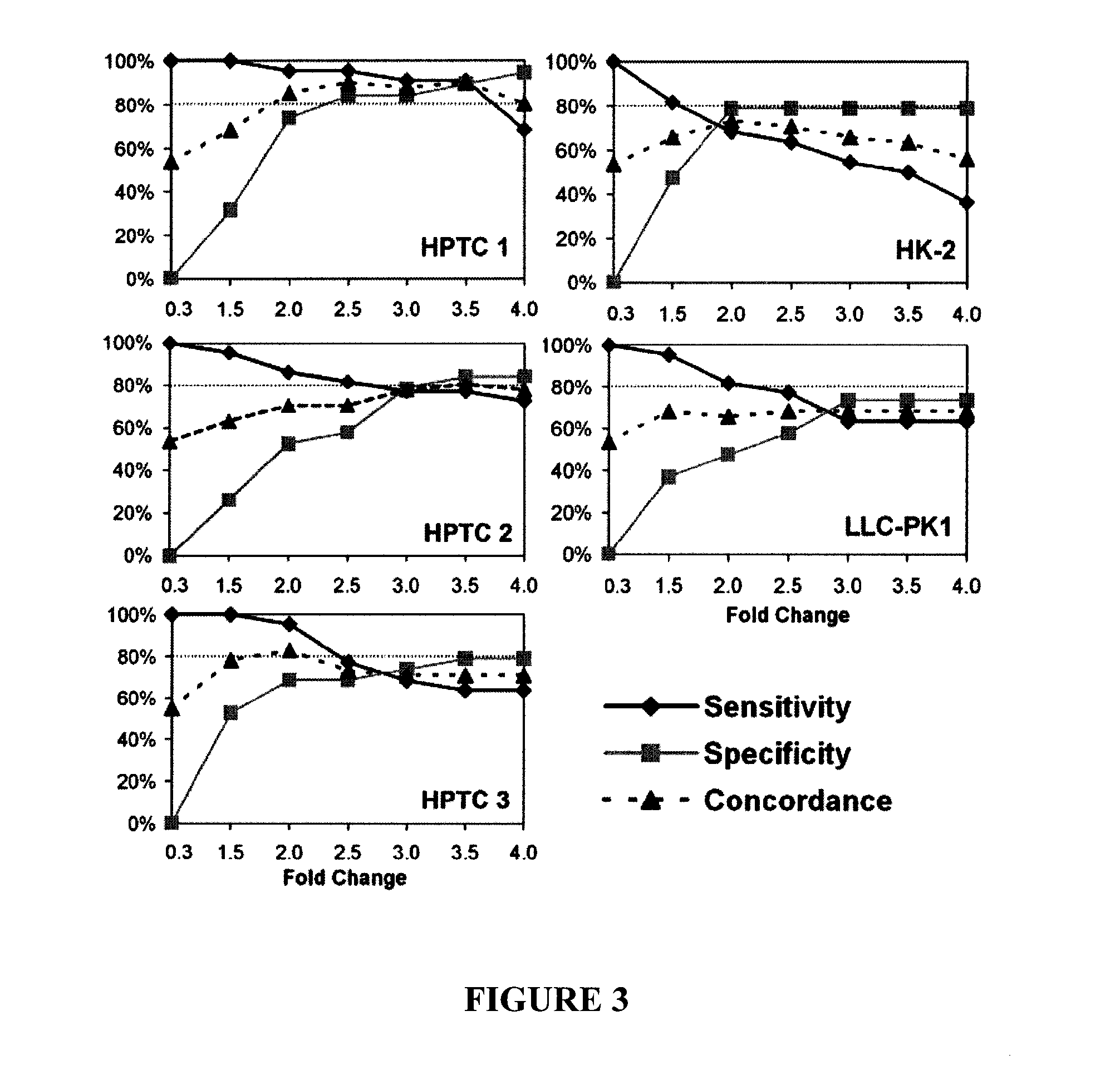
In vitro assay for predicting renal proximal tubular cell toxicity
InactiveUS20150197802A1Save a lot of costEarly detectionMicrobiological testing/measurementLibrary screeningInterleukin 6Interleukin 8
There is provided an in vitro assay for screening a test compound for toxicity in renal proximal tubular cells. The method comprises contacting a test compound with a test population of renal proximal tubular cells; and determining the expression level of an interleukin in the test population, the interleukin being interleukin-6 (IL-6) or interleukin-8 (IL-8), or both. Expression levels of the interleukin in the test population being greater than expression levels in a control population of renal proximal tubular cells not contacted with the test compound is indicative that the test compound is toxic for renal proximal tubular cells.
Owner:AGENCY FOR SCI TECH & RES
Method promoting conception by administering IL-8 or MCAF
InactiveUS6013252ARaise the possibilityPromote growthBiocidePeptide/protein ingredientsInterleukin 8Ovum implantation
PCT No. PCT / JP96 / 02412 Sec. 371 Date Jun. 5, 1997 Sec. 102(e) Date Jun. 5, 1997 PCT Filed Aug. 28, 1996 PCT Pub. No. WO97 / 07813 PCT Pub. Date Mar. 6, 1997Leukocyte chemotactic factors such as interleukin-8 and MCAF, and inductive substances therefor were revealed to have proconceptive activities. These activities include promoting ovum growth and fertilized ovum implantation, and are exhibited by a sole substance. Accordingly, it has been shown that such substances can be used in drugs concerning medical treatment for infertility, and further, can be used in veterinary and livestock industry fields such as reproduction of industrial animals or species preservation of rare animals.
Owner:TORAY IND INC
Application of mi-RNA (micro-ribonucleic acid) with AAAGUGC seed sequence in preparing interleukin 8 inhibitor
The invention discloses an application of mi-RNA (micro-ribonucleic acid) with an AAAGUGC seed sequence in preparing an IL8 (interleukin 8) inhibitor. The mi-RNA with the AAAGUGC seed sequence can directly target the 3' non-coding region of the mRNA of IL8, so that secretion of IL8 can be effectively inhibited, and generation of cell endogenic IL8 can be reduced. Therefore, the mi-RNA with the AAAGUGC seed sequence can be used as a cell endogenic IL8 generation inhibitor and used for preventing or treating various diseases or disease states caused by IL8 generation or increase.
Owner:DALIAN MEDICAL UNIVERSITY
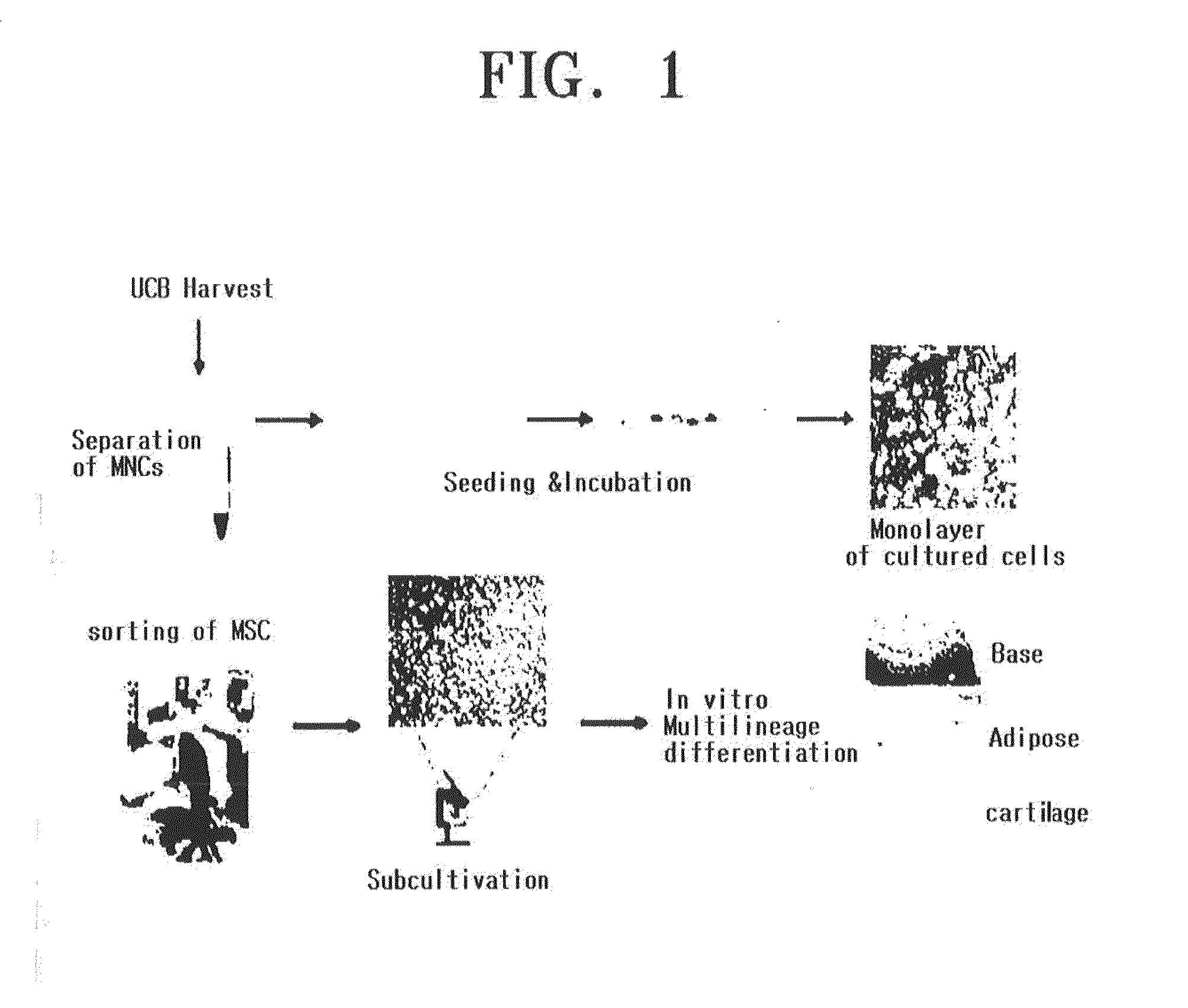
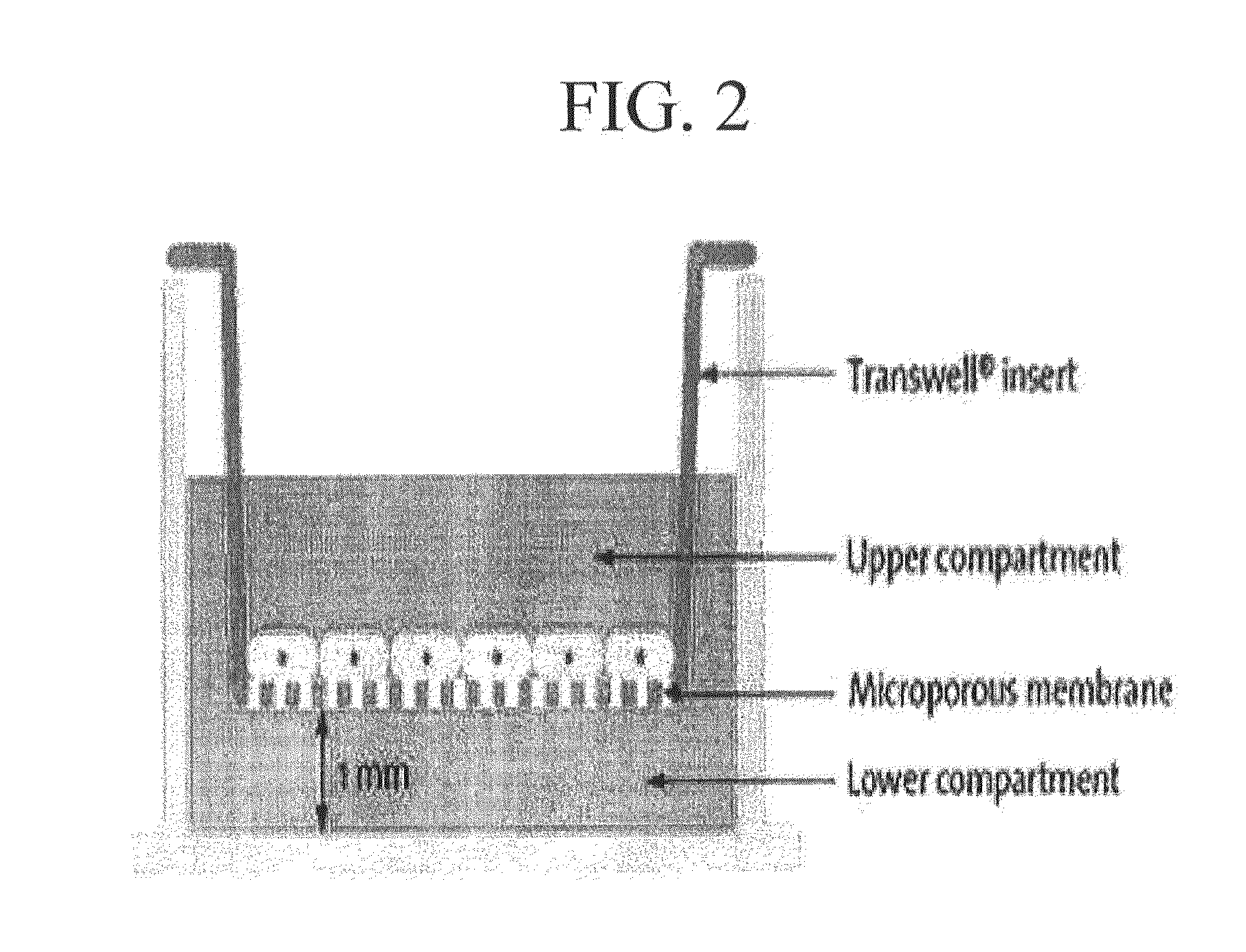
COMPOSITION FOR THE DIAGNOSIS, PREVENTION OR TREATMENT OF DISEASES RELATED TO CELLS EXPRESSING IL-8 OR GRO-ALPHA, COMPRISING UCB-MSCs
InactiveUS20130189189A1Induce the tropism of UCB-MSCsEfficient deliveryUltrasonic/sonic/infrasonic diagnosticsBiocideDiseaseInterleukin 8
Owner:MEDIPOST
Composition for promoting physiologically regulative regeneration of damaged tissue as well as preparation method and use thereof
ActiveCN104056258AReturn to normal structureRestore physical functionPeptide/protein ingredientsDermatological disorderTissue repairDamages tissue
The invention discloses a composition for promoting physiologically regulative regeneration of a damaged tissue. The composition comprises such components as albumin, epidermal growth factor, transforming growth factor alpha, keratinocyte growth factor, basic fibroblast growth factor, platelet-derived growth factor, vascular endothelial growth factor, interleukin-8 and granulocyte-macrophage colony stimulating factor. The eight protein factors are combined for use to comprehensively develop the physiological tissue repair function; the eight protein factors functionally promote each other; based on the own physiological tissue recovery mechanism of the human body, the wound healing is accelerated and the wound healing quality is improved, including recovering the structure and the functions of the normal skin tissue. Each cell factor-containing component has clear mechanism of action without any potential toxic and side effect and objectionable odor. Such a combined recovery agent is capable of recovering the normal structure and the physiological functions of tissues more quickly under the physiological conditions of the human body.
Owner:高忠翔
Human monoclonal antibodies against interleukin 8 (IL-8)
InactiveUS7622559B2More therapeutically effectiveLess immunogenicNervous disorderAntipyreticV(D)J recombinationInterleukin 8
Isolated human monoclonal antibodies which bind to IL-8 (e.g., human IL-8) are disclosed. The human antibodies can be produced in a hybridoma, transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals, hybridomas, and transfectomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:CORMORANT PHARMA
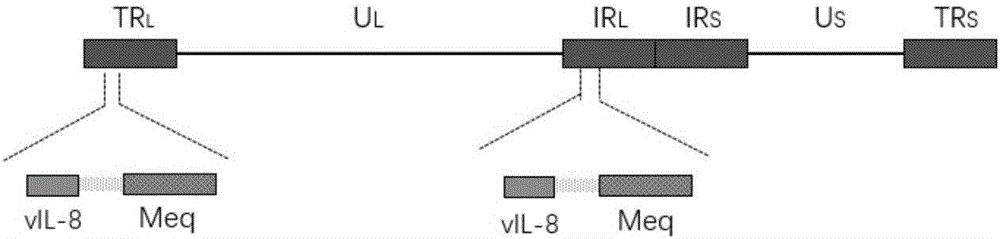
Construction of recombinant MDV (Marek's Disease Virus) and vIL-8 (Viral Interleukin 8) double-gene deleted strain and application thereof
ActiveCN106754761AGood immune protectionViral antigen ingredientsVirus peptidesImmune effectsSerum ige
The invention provides a recombinant serum I type MDV (Marek's Disease Virus) (a BZ-1 strain). The recombinant serum I type MDV is actually characterized in that pathogenic associated genes (meq and vIL-8 (Viral Interleukin 8) genes) of an isolated MDV virulent strain BJ07 are knocked out at the same time, and a constructed MDV gene deleted strain (the BZ-1 strain) has no pathogenicity, cannot induce tumor of chicken and has no immunosuppressive action on chicken flocks; the BZ-1 strain is used as a vaccine prepared by a strain produced by MD (Marek's Disease) live vaccine, chicken MD induced by a very virulent MDV can be prevented, and the protective immune effect is superior to that of a CVI988 / Rispens strain vaccine which is most widely applied in domestic and foreign markets at present.
Owner:北京邦卓生物科技有限公司
Breeding method of new disease-resistance large white pig strain
InactiveCN106973854AEnhance immune responseReduce morbidityAnimal feeding stuffBiological testingInterleukin 6Lean meat
The invention discloses a breeding method of a new disease-resistance large white pig strain. The method includes the following steps that 1, a feed adding method is adopted to conduct an Escherichia coli infection experiment on weaned piglets of a core group in large white pigs, and an Escherichia coli resistant breeding basic group in the large white pigs is established; 2, concentration determination is conducted on cytokines of interleukin 1beta, interleukin 4, interleukin 6, interleukin 8, interleukin 10, transforming growth factor beta, tumor necrosis factor alpha and interferon gamma of an Escherichia coli resistant population in the basic group; reproductive performances are detected and selected, growth speeds, lean meat ratios and carcass quality characters, and a first generation is established; 3, a group subculture selective breeding method and a molecular-marker-assisted selection method are adopted. In this way, by the adoption of the breeding method of the new disease-resistance large white pig strain, after four to five generations, a vested breeding target is achieved gradually, a new-strain core group is established, and the morbidity of piglet diarrheal diseases is expected to be reduced.
Owner:TAICANG JINZHU AGRI DEV
Composition for treatment of CXCL8-mediated lung inflammation
InactiveCN102596227AHigh binding affinityElevated GPCR activityPeptide/protein ingredientsAntipyreticObstructive Pulmonary DiseasesARDs - Acute respiratory distress syndrome
Owner:PROTAFFIN BIOTECHNOLOGIE AG
Use of interleukin 17E for the treatment of cancer
InactiveUS8287851B2Growth inhibitionImprove the level ofOrganic active ingredientsSugar derivativesInterleukin 10Nucleotide
Owner:APTOSE BIOSCIENCES INC
Cancer cell migration inhibitors and their use in therapeutic treatments
InactiveUS20170165363A1Reduce probabilityFunction increaseBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsInterleukin 6Cancer cell
Described are methods of treating or preventing cancer in patients by administering Interleukin 6 (IL-6) inhibitor and Interleukin 8 (IL-8) inhibitor, in a concentration ratio range to inhibit the migration of cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
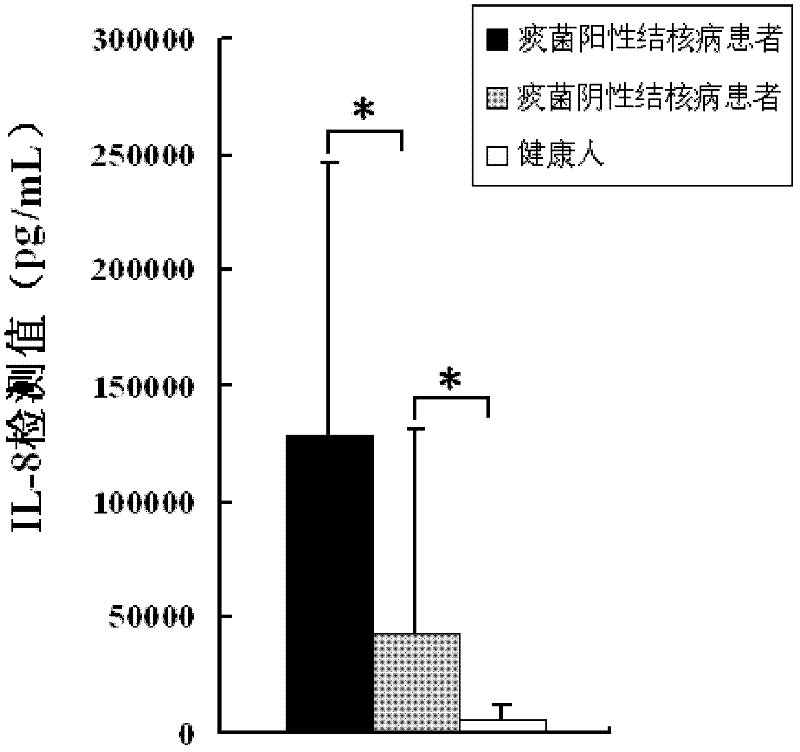
Kit for auxiliary diagnosis of tuberculosis patients
InactiveCN102590502AImprove securityImprove practicalityMaterial analysisWhite blood cellDisease course
The invention discloses a kit for auxiliary diagnosis of tuberculosis patients. The kit comprises total protein of Mycobacterium tuberculosis and a substance for detecting interleukin-8. After the peripheral blood of patients to be tested is induced by using the total protein of the Mycobacterium tuberculosis, the expression levels of the interleukin-8 (IL-8) are remarkably different between tuberculosis patients and healthy person, among tuberculosis patients with different degrees of severity, and among different disease course stages of the same tuberculosis patient; and the kit can be used for the auxiliary diagnosis of the tuberculosis patients through the difference of the expression levels (the diagnosis comprises the diagnosis of the tuberculosis patients with different degrees of severity, the diagnosis of the different disease course stages of the same tuberculosis patient, and the diagnosis for cure of the tuberculosis patients).
Owner:BEIJING TUBERCULOSIS & THORACIC TUMOR RES INST
Method for inducing in vivo migration of stem cell
The present invention relates to an implantable composition for treating a damaged tissue and a method for inducing an in vivo migration of a cell for treatment to a damaged tissue region. The present invention treats the damaged tissue by inducing / promoting homing of a cell for tissue generation by implanting a biodegradable scaffold reacted with chemotactic factors (for example, IL-8 or MIP-3±) to a damaged location (for example, joint cartilage or skin). Thus, the composition of the present invention can not only be applied to the treatment of a damaged bone tissue, a joint cartilage, or a skin tissue more conveniently and efficiently compared to the conventional technology, but can also be used as a useful treatment supplement agent in cell treatment using allogeneic cell by enabling efficient utilization of cell resources for treatment, the cell resources which are high in scarcity.
Owner:TEGO SCI
Method for screening probiotics
The invention discloses a method for screening probiotics. The method comprises the following steps of: (1) carrying out a primary culture on spleen tissues of a pig or a domestic animal; (2) attacking toxicity of the tissues through colon bacillus K88, and testing variations of levels of an interleukin 8 and an interleukin 4; (3) respectively processing the tissues through candidate probiotic strains, and testing variations of levels of two interleukins; (4) comparing the variations to select probiotic strains which are similar in variation degrees of the two interleukins but opposite in effect due to treatment of the colon bacillus K88; and (5) through the colon bacillus K88 animal toxicity attacking test, observing protecting effects of primarily selected probiotic strains, and finally determining an efficient probiotic strain. The method for screening the probiotic disclosed by the invention has the remarkable advantages that: the method combining in vitro immunology experiment and in vivo toxicity attacking experiment is high in accuracy, strong in pertinency and time-saving; and the screened probiotics can effectively replace antibiotic, and greatly reduce diseases caused by colon bacillus so as to promote the health of the animal.
Owner:江西华农恒青农牧有限公司
Silicon-containing biodegradable material for Anti-inflammatory therapy
A method for preventing and / or treating a disease associated with increased interleukin-1β and / or interleukin-6 and / or interleukin-8 activity and / or disease, in which a reduction in the activity of interleukin-1β and / or interleukin-6 and / or interleukin-8 is beneficial for healing includes utilizing a silicon-containing, biodegradable material containing a polyhydroxysilicic acid ethyl ester compound, with the proviso that wound defects including chronic diabetic-neuropathic ulcer, chronic leg ulcer, bedsores, secondary-healing infected wounds, non-irritating, primary-healing wounds, ablative lacerations and / or abrasions, are excluded from said disease that is prevented and / or treated with the silicon-containing, biodegradable material.
Owner:JIANGSU SYNECOUN MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com