Medicine for relieving severe asthma, application and animal model construction method
A technology of animal model and construction method, which is applied in the direction of medical formula, drug combination, and medical preparations containing active ingredients, etc., and can solve problems such as unclear mechanism of action, unclear pathogenesis of severe asthma, and difficult clinical treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] In order to make the objectives, technical solutions and advantages of the present invention, the present invention will be further described in detail below with reference to the embodiments. It should be understood that the specific embodiments described herein are merely intended to illustrate the invention and are not intended to limit the invention.
[0030] For the problems of the prior art, the present invention provides a method of alleviating a medicament, application, and animal model construct of severe asthma, and a detailed description of the invention will be described in connection with the accompanying drawings.
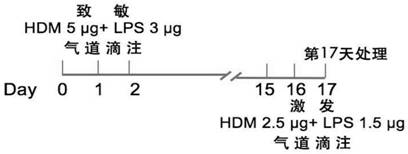
[0031] Such as figure 1 As shown, the animal model construction method provided by the present invention includes the following steps:
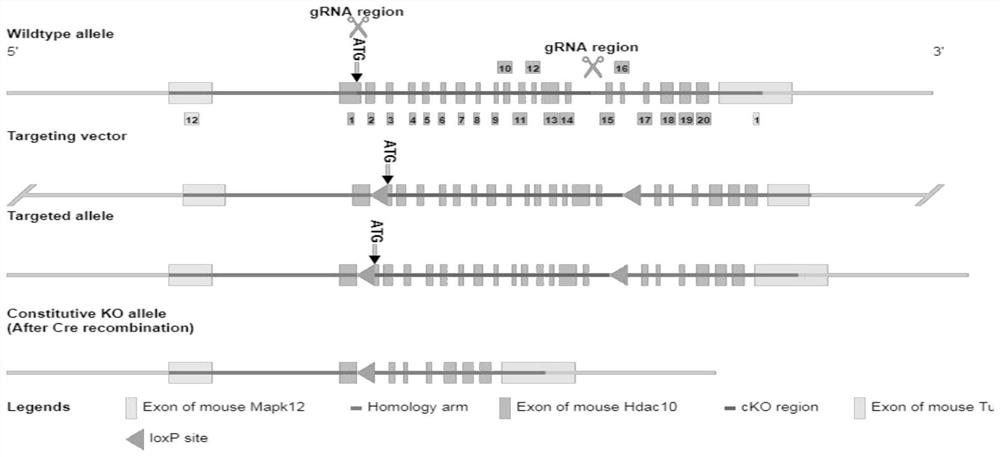
[0032] S101: Using HDAC10 lymphocyte condition knockout (HDAC10 fl / fl Constructs astatic asthma model in mice. The total number of cells were detected by mice AHR and alveolar lavewater (BALF) was detected by a to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com