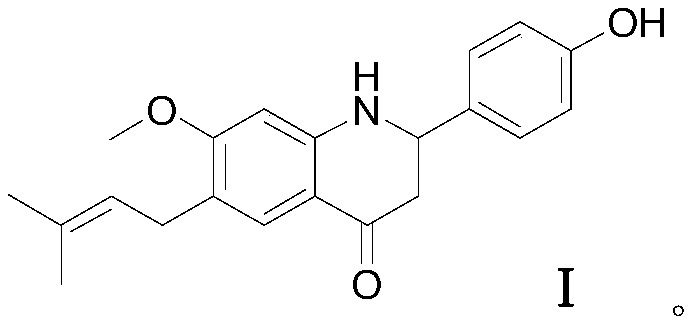
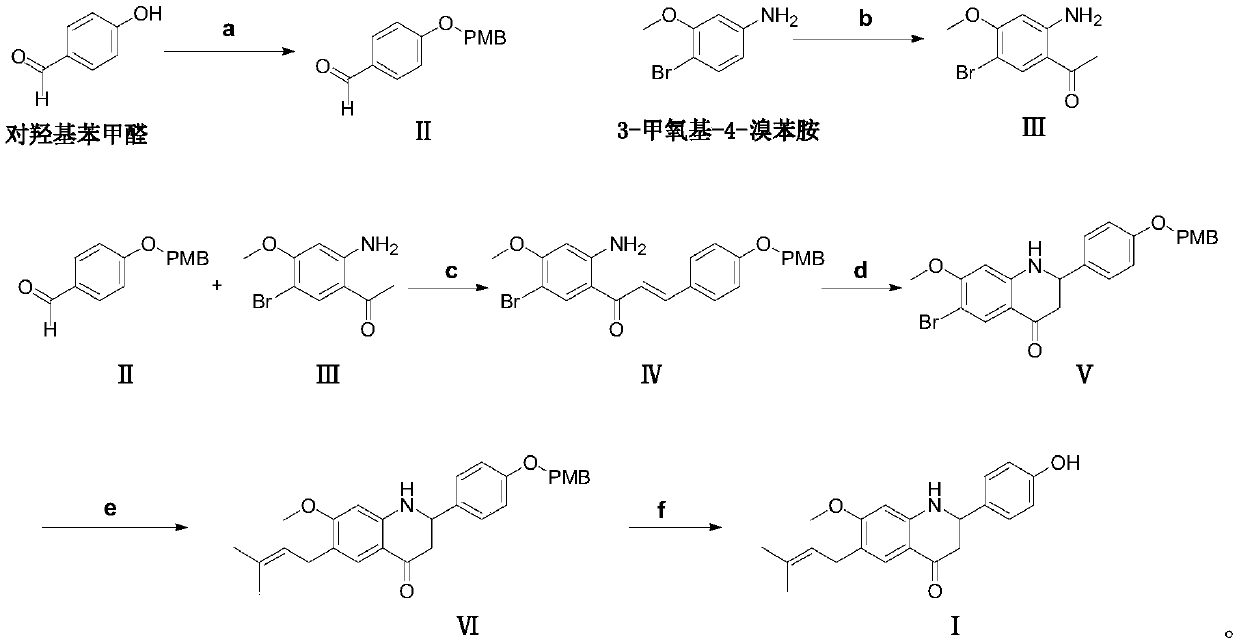
A compound with ppar multiple agonistic activity and its preparation method and application
A compound and active technology, applied in the field of chemical medicine, can solve problems such as single effect of hypoglycemic or lipid-lowering drugs, inability to regulate glucose and lipid metabolism at the same time, and linking clinical diseases, etc., to achieve wide application prospects and significant medicinal value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
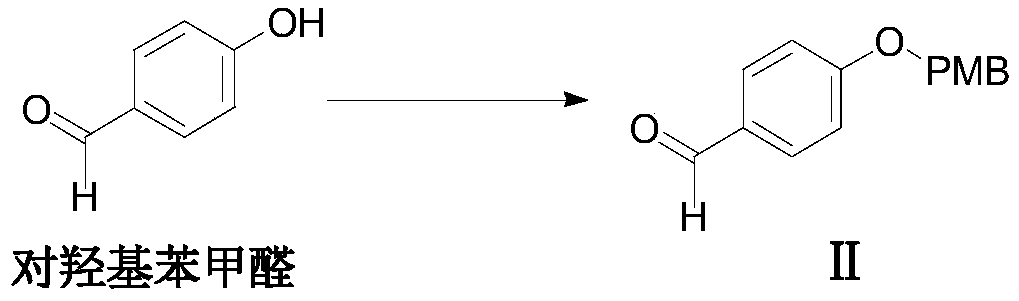
[0053] Embodiment 1: the preparation of formula II compound
[0054]
[0055] Add p-hydroxybenzaldehyde (3.7g, 30.0mmol) and potassium carbonate (12.4g, 90.0mmol) into DMF (40mL), stir and dissolve at room temperature, slowly drop p-methoxybenzyl chloride (3.5g, 30.0 mmol), react at room temperature (about 20-25° C.) for 12 hours, end the reaction, add saturated NaCl solution to quench the reaction, then add ethyl acetate for extraction until the extraction is complete, combine the organic phases, and dry the organic phases with anhydrous sodium sulfate. Suction filtration, concentrated under reduced pressure until no solution distilled off to obtain a white powder substance, which is the compound of formula II (named: 4-p-methoxybenzylhydroxy-benzaldehyde): 6.2g, 25.6mmol, molar yield was 85.3%.
Embodiment 2
[0056] Embodiment 2: the preparation of formula III compound
[0057]
[0058] Dissolve 3-methoxy-4-bromoaniline (6.0g, 30.0mmol) in dichloromethane (30mL), cool to 0°C in an ice bath, and slowly add BCl dropwise at 0°C 3 Dichloromethane solution (1.0mol / L, 33mL), keep stirring for half an hour, then slowly add AlCl 3 (4.4g, 33.0mmol) and CH 3 CN (3.2mL, 60.0mmol), after the addition is complete, stir the reaction at room temperature for half an hour, then rise to 70°C and keep the temperature for 12 hours, and then end the reaction. After the reaction solution is cooled, add 2N HCL to quench the reaction (the process of adding hydrochloric acid The reaction solution will have a lot of white bubbles, try to add it slowly in an ice bath at 0°C), then stir at 70°C for 2 hours, after cooling to room temperature, add 1N NaHCO 3 Adjust the solution to neutral, then add dichloromethane to extract until the extraction is complete, combine the organic phases, dry the organic phas...
Embodiment 3
[0061] Embodiment 3: the preparation of formula IV compound
[0062]
[0063] Add the compound of formula II (1.7g, 6.5mmol) and the compound of formula III (1.2g, 5.0mmol) into absolute ethanol (20mL), then add solid sodium hydroxide (800.0mg, 20.0mmol), after the addition is complete, the temperature is raised Reaction at 60°C for 12 hours, the reaction was terminated, the reaction solution was cooled to room temperature, filtered, the filter cake was washed with ethanol, and dried to obtain a yellow powder substance, which was the compound of formula IV (named: 1-(2'-amino-4 '-methoxy-5'-bromo)-3-(4'-methoxybenzylanisole)-2E-propen-1-one): 2.2 g, 4.66 mmol, and the molar yield is 93.2%.
[0064] Tested:
[0065] 1 H NMR (300MHz, CDCl 3 )δ8.00(s,1H,ArH),7.70(d,J=15.3Hz,1H,=H),7.59(d,J=8.4Hz,2H,ArH),7.44–7.33(m,3H,= H / ArH), 6.96(dd, J=21.1, 8.3Hz, 4H, ArH), 6.59(s, 2H, NH2), 6.13(s, 1H, ArH), 5.04(s, 2H, OCH 2 ),3.89(s,3H,ArOCH 3 ),3.82(s,3H,ArOCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com