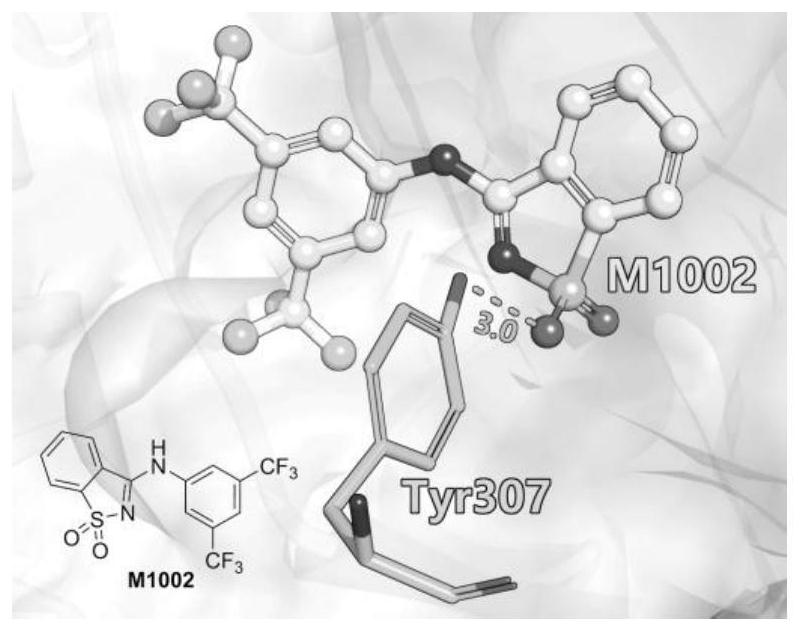
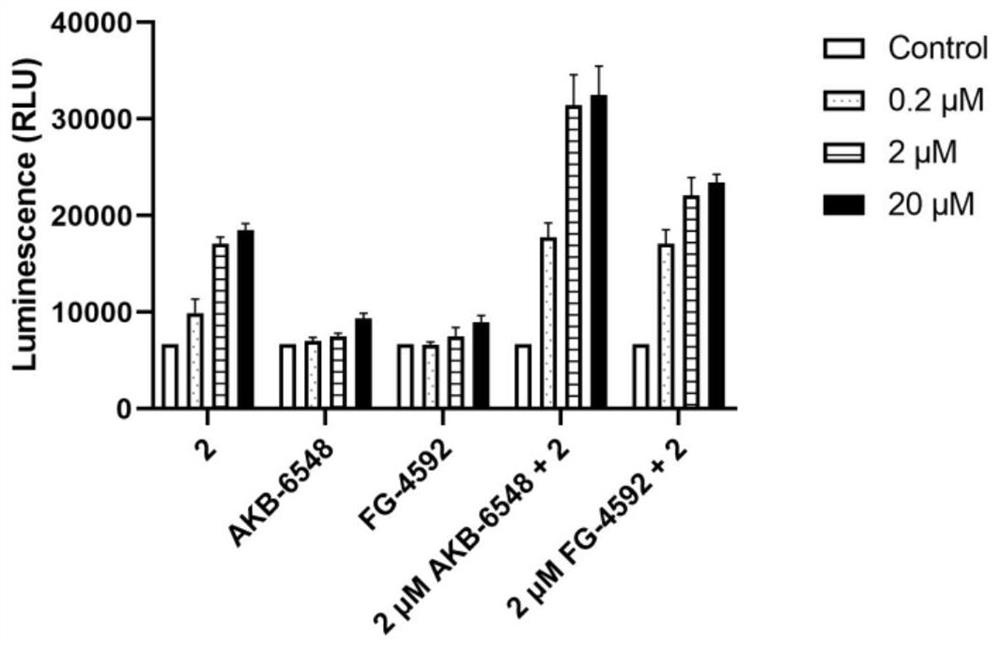
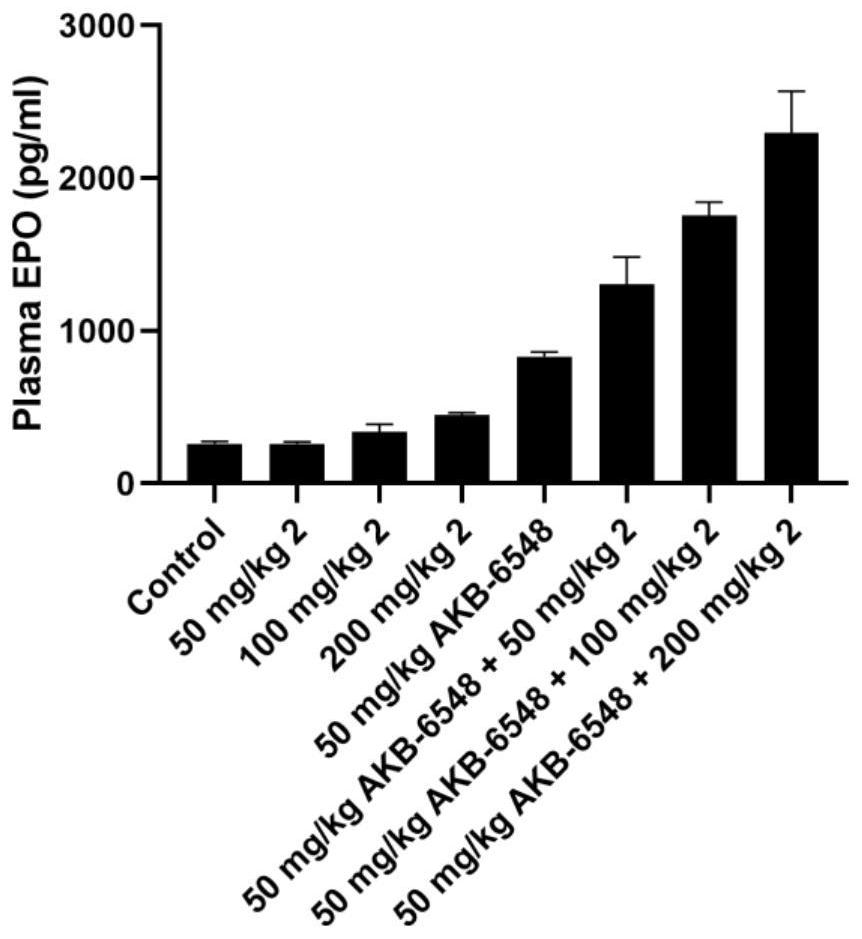
Benzisothiazole hypoxia-inducible factor 2 agonist compound or pharmaceutically acceptable salt, preparation method and application thereof
A hypoxia-inducible factor, benzisothiazole technology, applied in organic chemistry, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as weak activity, achieve high-efficiency EPO levels, avoid subtype selectivity, The effect of increasing EPO levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] N-(3,5-Dibromophenyl)benzo[d]isothiazol-3-amine (1)
[0046] (1) Preparation of 2-chloro-N-(3,5-dibromophenyl)benzamide
[0047] Add sodium hydride (31.3 mg, 1.2 mmol) and 3,5-dibromoaniline (276.0 mg, 1.1 mmol). After the reaction was stirred for 0.5 hours in an ice bath, the reaction gradually returned to room temperature, and the reaction was maintained at room temperature for 24 hours. After the reaction, the reaction solution was quenched by adding water (5 mL). The reaction solution was adjusted to pH ~ 3.0 with dilute hydrochloric acid, the target product was protonated and dissolved in the water phase, and the water phase was washed with dichloromethane (3×10 mL) to remove impurities in the organic phase. The aqueous phase was adjusted to pH~7.0 with saturated sodium bicarbonate solution and extracted with dichloromethane (3 x 10 mL). The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduc...
Embodiment 2
[0051]
[0052] N-(3,5-dimethoxyphenyl)benzo[d]isothiazol-3-amine (2)
[0053] (1) Preparation of 2-chloro-N-(3,5-dimethoxyphenyl)benzamide
[0054] In an ice bath, 3,5-dimethoxyaniline (1.23g, 8.0 mmol) and sodium hydride (0.524 g, 22.0 mmol). React at room temperature for 1 hour. After the reaction, the reaction solution was quenched by adding ice water (25 mL). The reaction solution was adjusted to pH ~ 3.0 with dilute hydrochloric acid. Dichloromethane (3 x 10 mL) was added to wash the aqueous phase to remove impurities from the organic phase. The aqueous phase was adjusted to pH ~ 7.0 with saturated sodium bicarbonate solution and extracted with dichloromethane (3 x 10 mL). The organic phases were combined, and the solvent was distilled off under reduced pressure to obtain a red oily product. The next reaction was carried out directly without further purification.
[0055] (2) Preparation of N-(3,5-dimethoxyphenyl)benzo[d]isothiazol-3-amine
[0056] Dissolve 2-...
Embodiment 3
[0058]
[0059] N-(3,5-Dimethylphenyl)benzo[d]isothiazol-3-amine (3)
[0060] (1) Preparation of 2-chloro-N-(3,5-dimethylphenyl)benzamide
[0061]Add sodium hydride (31.3 mg, 1.2 mmol) and 3,5-dimethylaniline ( 133.3 mg, 1.1 mmol). The reaction solution was reacted in an ice bath for 0.5 hours, then returned to room temperature, and kept at room temperature for 24 hours. After the reaction, the reaction solution was quenched by adding water (5 mL). The reaction solution was adjusted to pH ~ 3.0 with dilute hydrochloric acid. Dichloromethane (3 x 10 mL) was added to wash the aqueous phase to remove impurities from the organic phase. The aqueous phase was adjusted to pH ~ 7.0 with saturated sodium bicarbonate solution and extracted with dichloromethane (3 x 10 mL). The organic phases were combined, and the solvent was distilled off under reduced pressure to obtain a brown oily crude product, which was directly used in the next reaction without further purification.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com