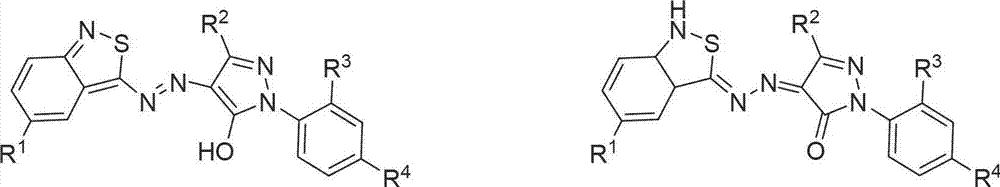Patents
Literature
209 results about "Isothiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An isothiazole, or 1,2-thiazole, is a type of organic compound containing a five-membered aromatic ring that consists of three carbon atoms, one nitrogen atom, and one sulfur atom. Isothiazole is a member of a class of compounds known as azoles. In contrast to the isomeric thiazole, the two heteroatoms are in adjacent positions.
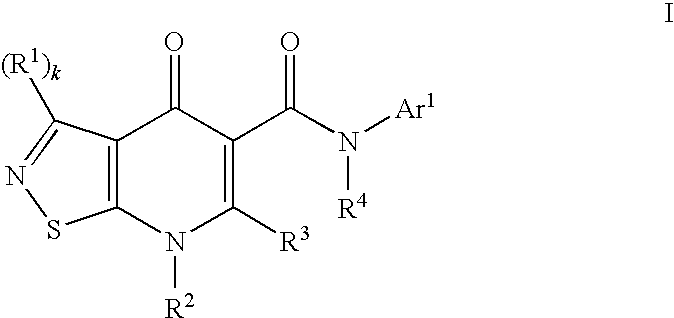
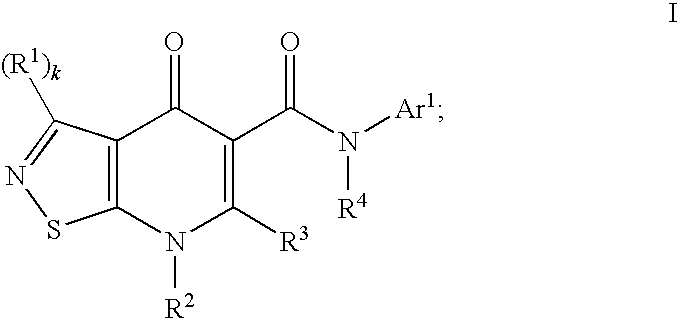
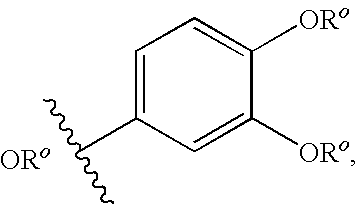
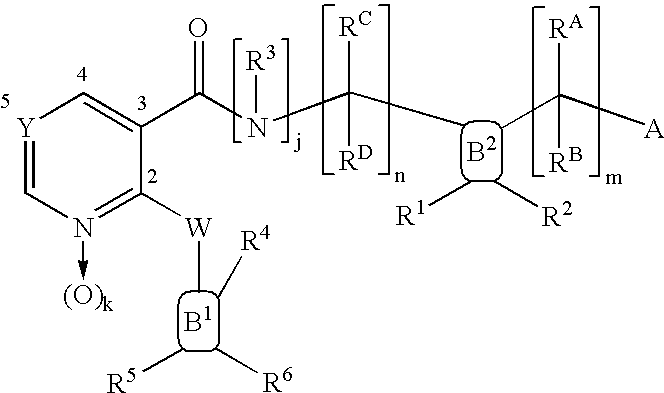
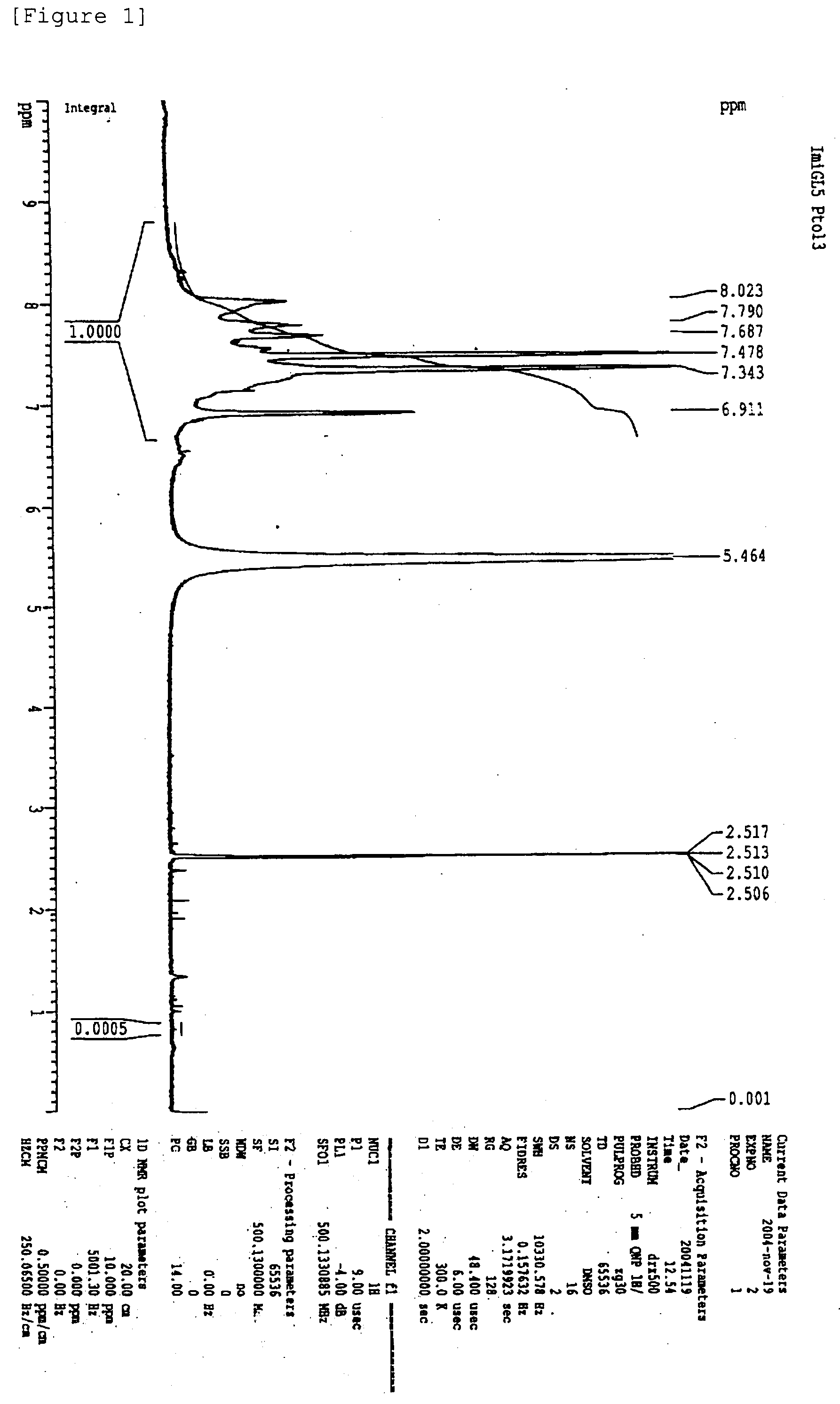
Isothiazolopyridinones useful for the treatment of (inter alia) cystic fibrosis
The present invention relates to modulators of ATP-Binding Cassette (“ABC”) transporters or fragments thereof, including Cystic Fibrosis Transmembrane Conductance Regulator, compositions thereof, and methods therewith. The present invention also relates to methods of treating ABC transporter mediated diseases using such modulators.
Owner:VERTEX PHARMA INC
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
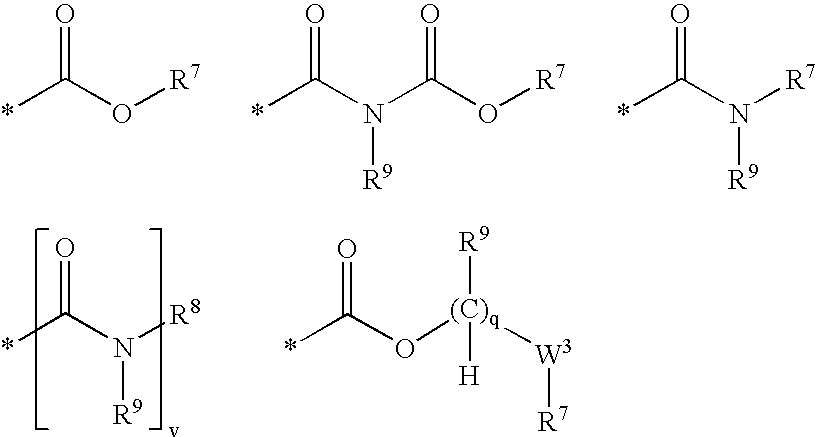
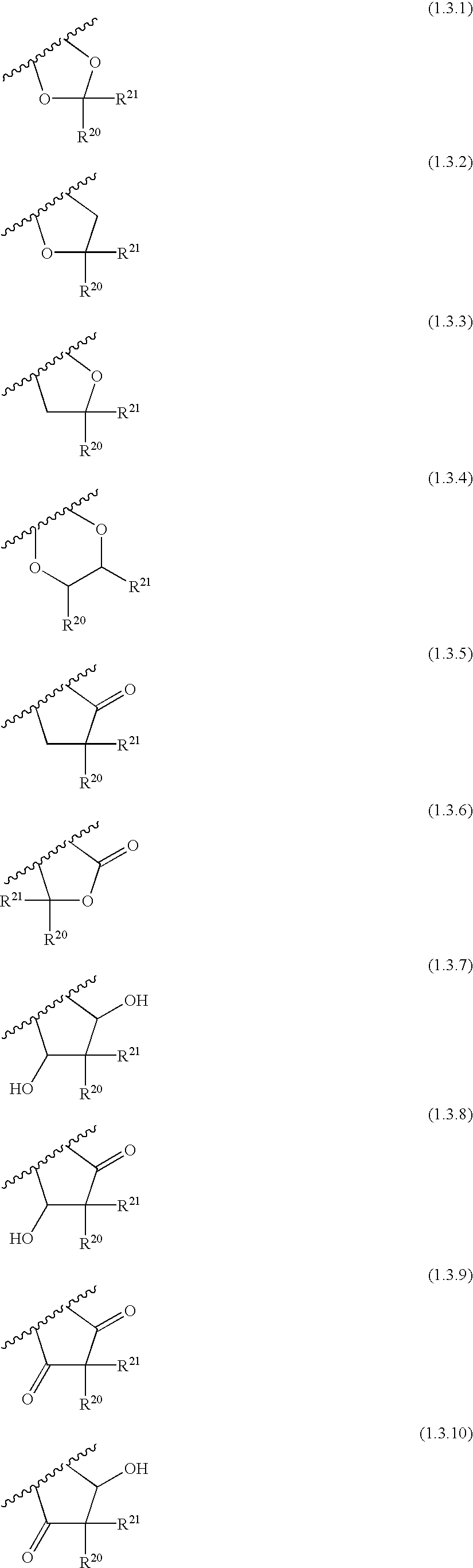
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Isoxazole-isoxazoles and isoxazole-isothiazoles
The present invention is concerned with isoxazole-isoxazoles and isoxazole-isothiazoles of formula I, having affinity and selectivity for GABA A α5 receptor, their manufacture, pharmaceutical compositions containing them and their use as cognitive enhancers or for the therapeutic and / or prophylactic treatment of cognitive disorders like Alzheimer's disease.
Owner:ROCHE PALO ALTO LLC
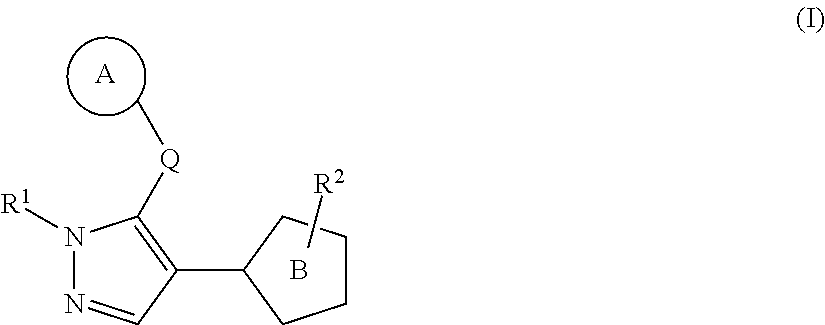
Heterocyclic compound
InactiveUS20140163001A1Enhanced inhibitory effectImprove efficacyBiocideNervous disorderThiazoleCombinatorial chemistry
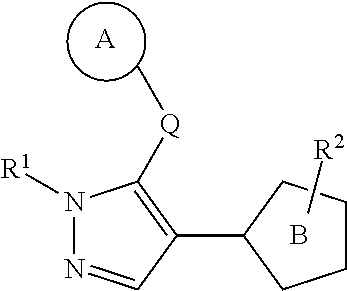
Provided is a heterocyclic compound having an RORγt inhibitory activity. A compound represented by the formula (I):whereinring A is an optionally substituted cyclic group,Q is a bond, optionally substituted C1-10 alkylene, optionally substituted C2-10 alkenylene, or optionally substituted C2-10 alkynylene,R1 is a substituent,ring B is a thiazole ring, an isothiazole ring or a dihydrothiazole ring, each of which is optionally further substituted by a substituent in addition to R2, andR2 is an optionally substituted cyclyl-carbonyl-C1-6 alkyl group, an optionally substituted aminocarbonyl-C1-6 alkyl group, an optionally substituted cyclyl-C1-6 alkyl group, an optionally substituted cyclyl-C1-6 alkylamino-carbonyl group, an optionally substituted aminocarbonyl-C2-6 alkenyl group, an optionally substituted C1-6 alkylcarbonylamino-C1-6 alkyl group, an optionally substituted cyclyl-aminocarbonyl group, an optionally substituted cyclyl-carbonyl group or an optionally substituted non-aromatic heterocyclic group, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
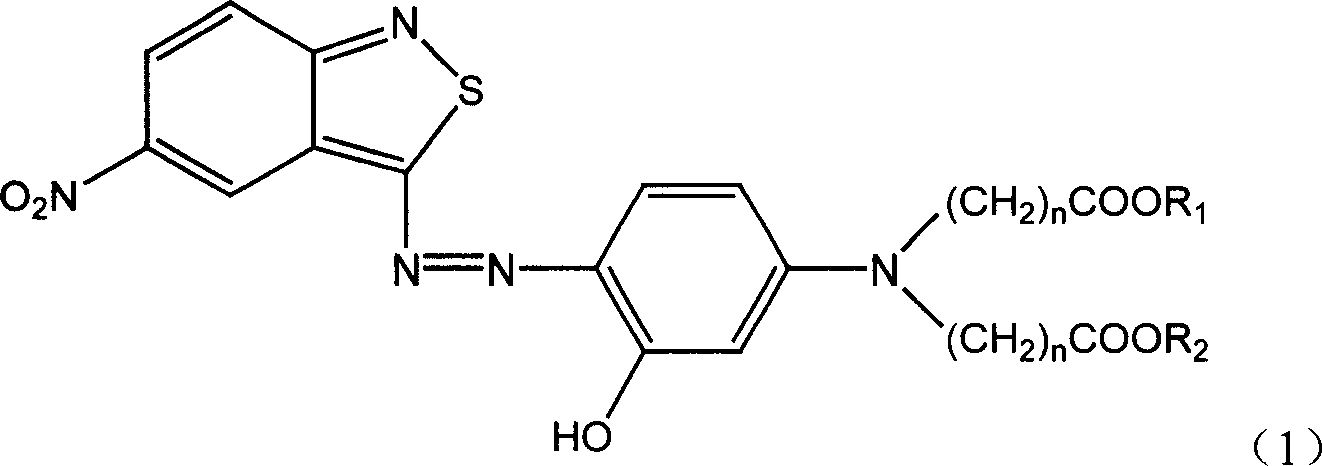
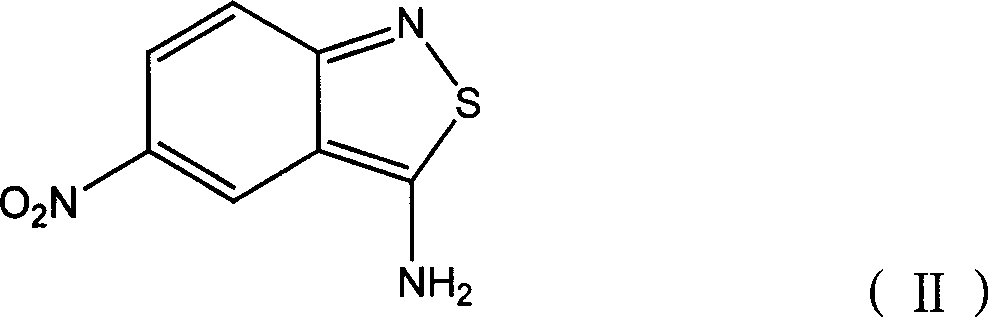
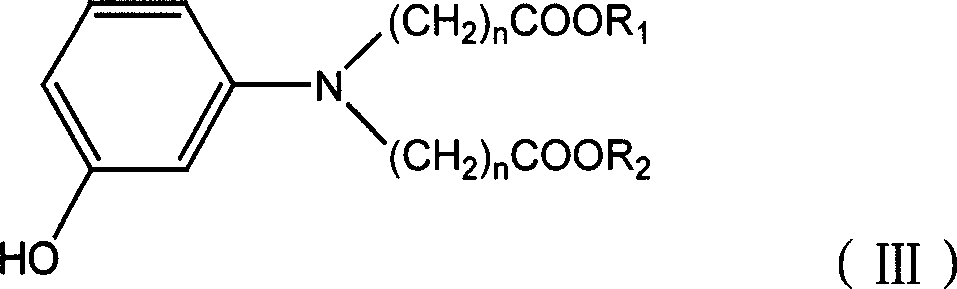
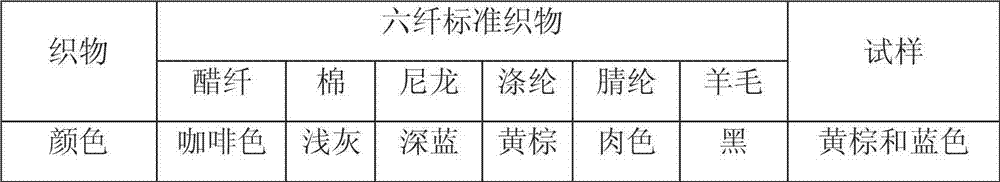
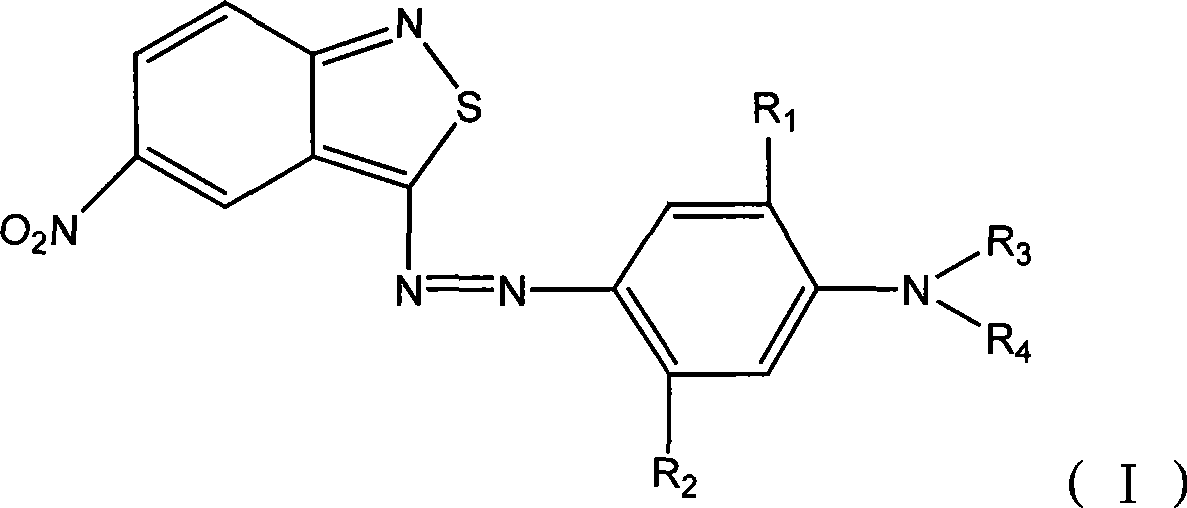
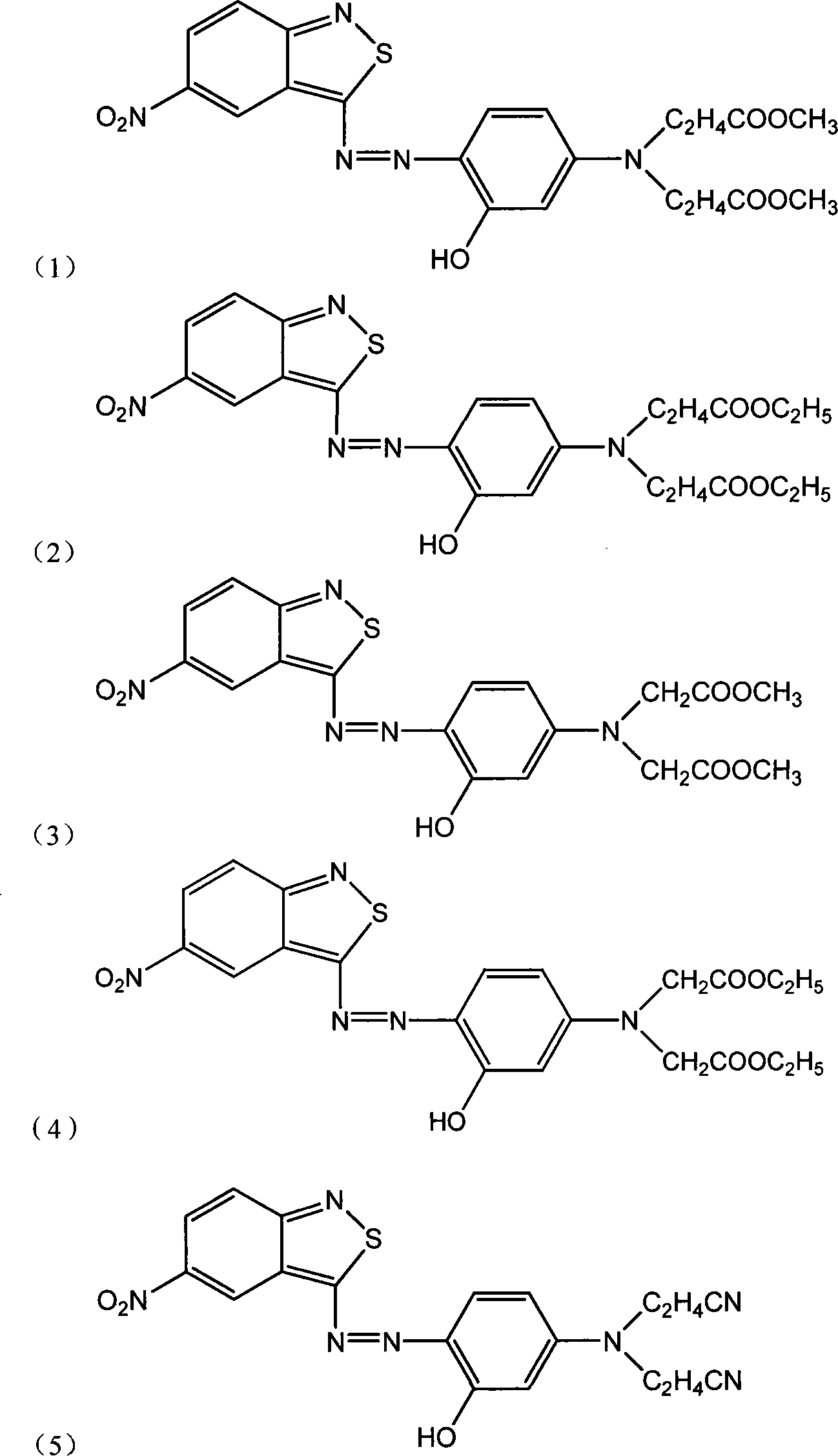
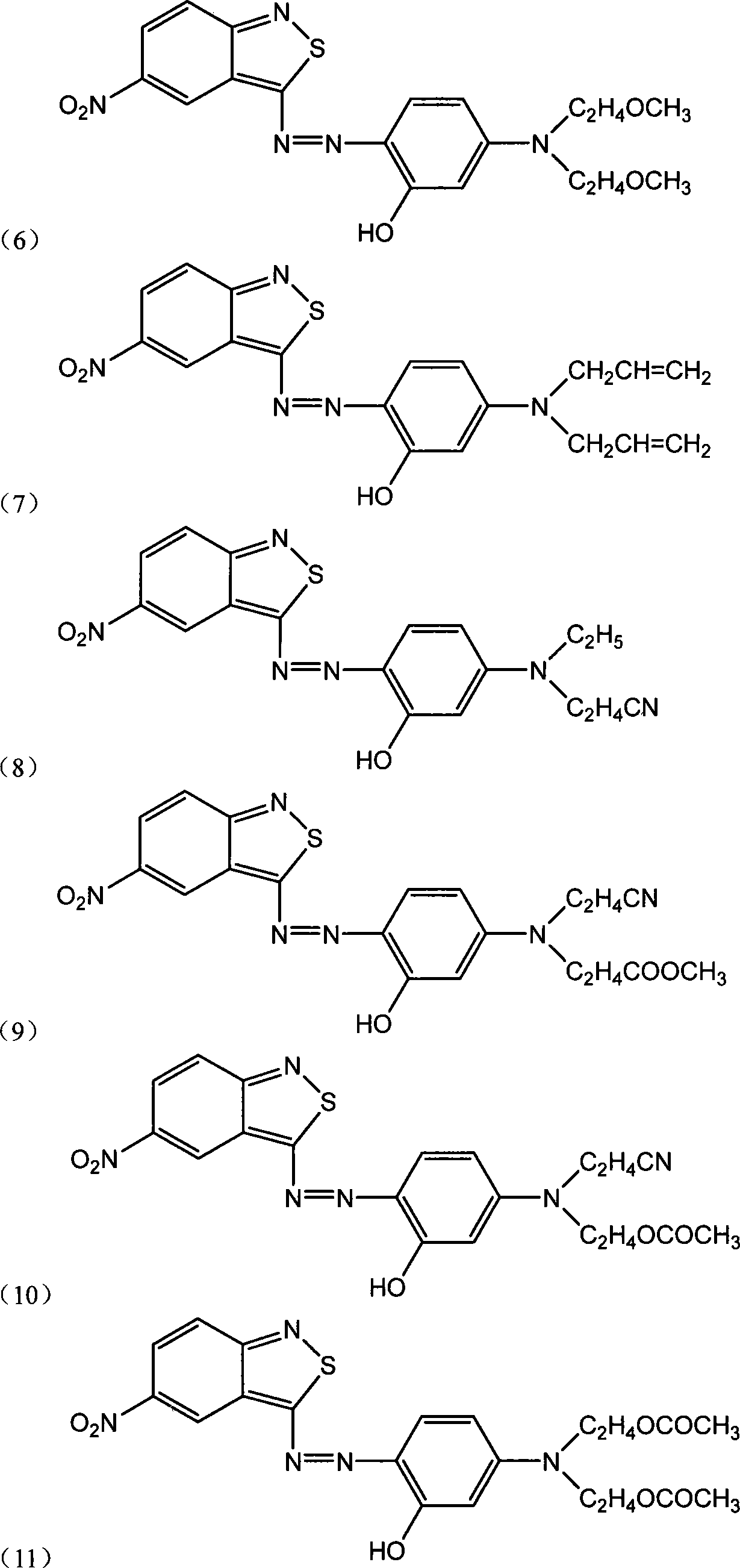
Benzo isothiazole compound, preparation and application and disperse dyes composition
ActiveCN101081838AImprove dyeing effectHigh color fastnessMonoazo dyesOrganic chemistryDisperse dyeIsothiazole
The present invention provides benzo isothiazoles as dye compounds in the structure as shown and their preparation, application and dye compositions. The dye compounds and their dye compositions are suitable for dyeing Dacron, acetate fiber, acrylon, polyester, mixed fiber and other hydrophobic fiber material, and possess excellent dyeing performances, high color fastness, low cost and broad application foreground.
Owner:ZHEJIANG LONGSHENG GROUP
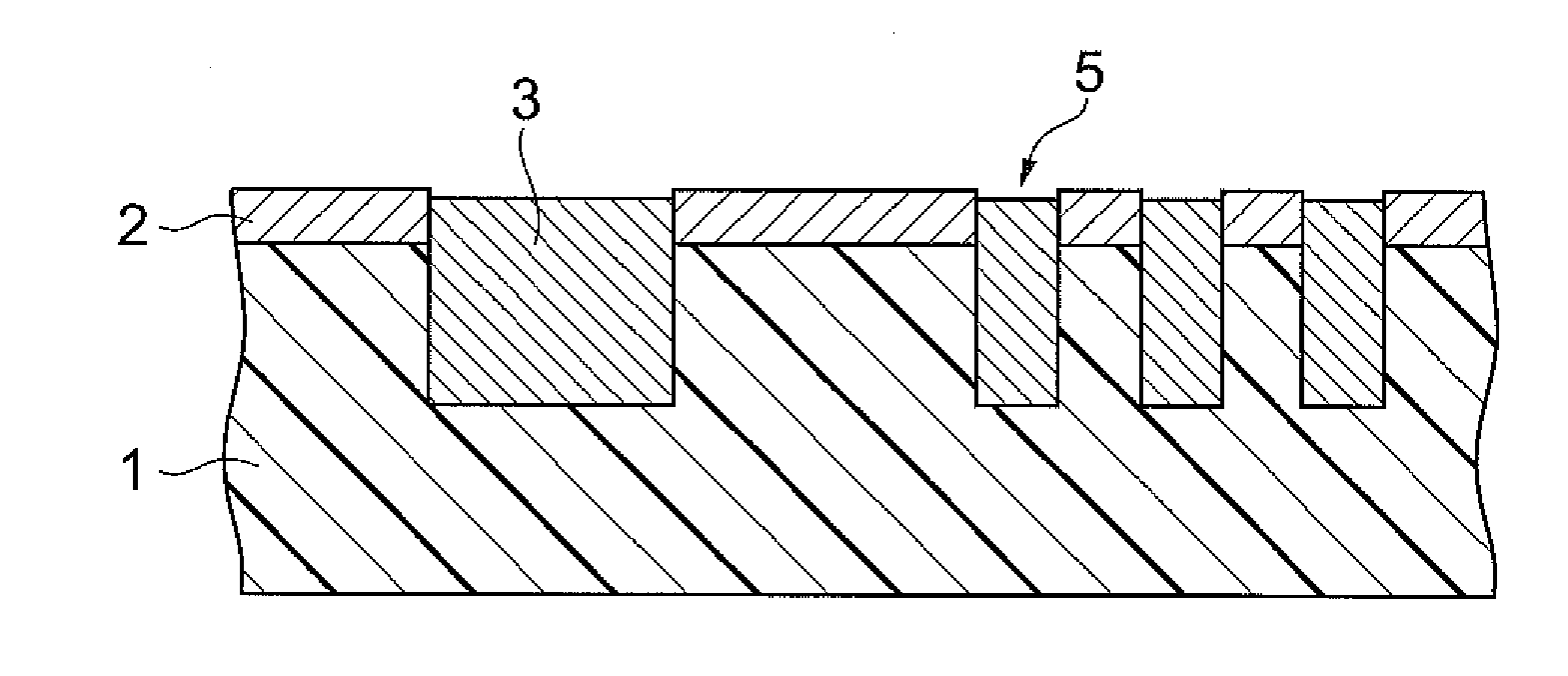
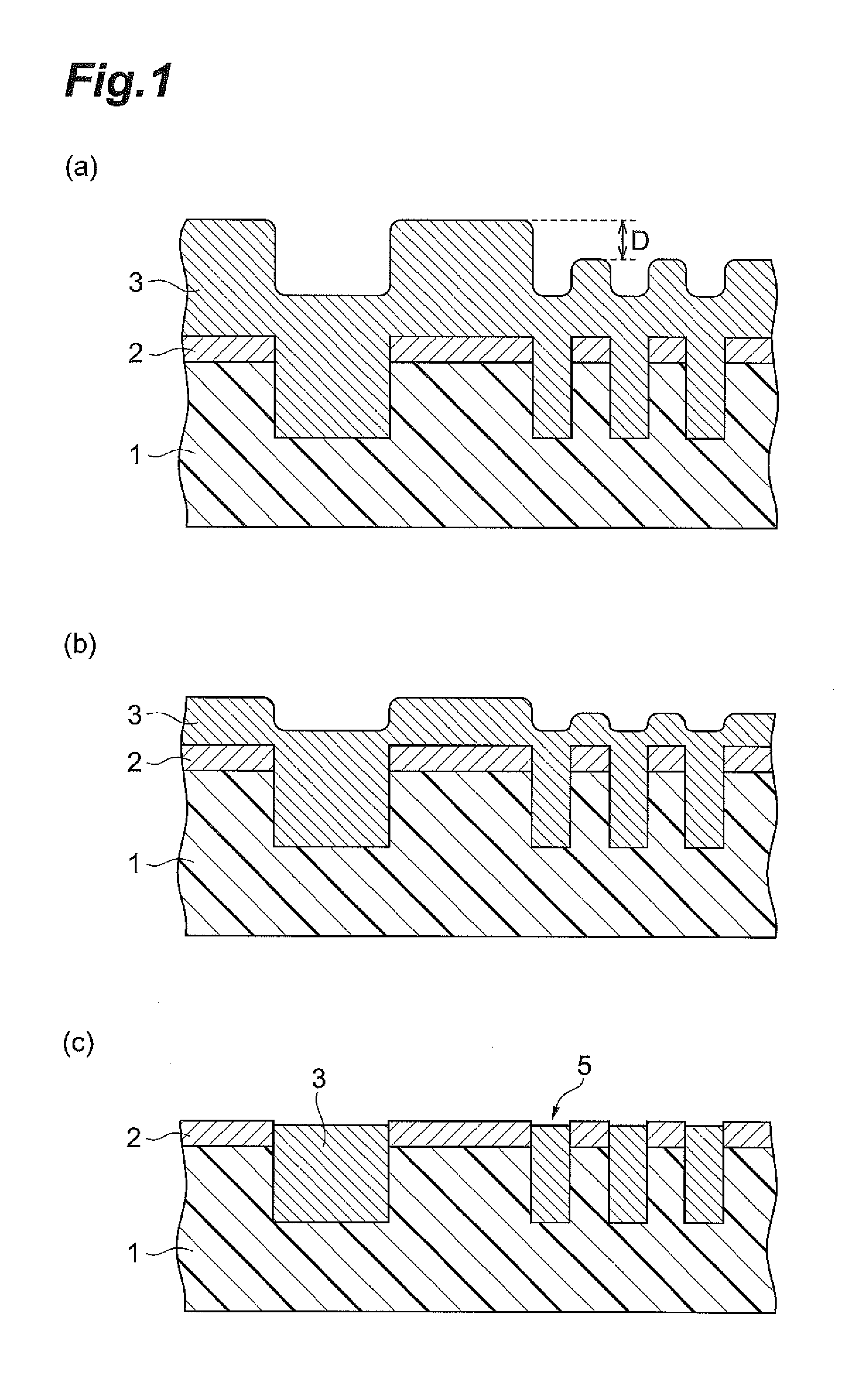
Polishing solution for cmp and polishing method using the polishing solution
ActiveUS20110275217A1Good water solubilitySatisfactory maintenance of dispersibilityOther chemical processesSemiconductor/solid-state device manufacturingMetallurgySilicon oxide
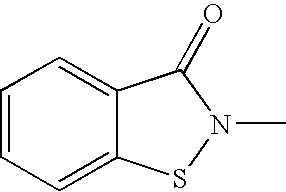
The polishing solution for CMP of the invention comprises abrasive grains, a first additive and water, wherein the first additive is at least 1,2-benzoisothiazole-3(2H)-one or 2-aminothiazole. The polishing method of the invention is a polishing method for a substrate having a silicon oxide film on the surface, and the polishing method comprises a step of polishing the silicon oxide film with a polishing pad while supplying the polishing solution for CMP between the silicon oxide film and the polishing pad.
Owner:RESONAC CORP
Bactericide for reverse osmosis membrane and method for preparing the same
InactiveCN1843589AExcellent bactericidal and algae killing effectImproved ability to kill bacteria and algaeBiocideSemi-permeable membranesIsothiazolinoneNitro compound
The invention relates to a disinfectant used in anti-penetrate film and relative preparing method. It is characterized in that: said disinfectant is formed by C4H4CLNOS C4H5NOS, bromine nitrogen isothiazole, bromine nitro compound, solvent and water. In the room temperature that under 50Deg. C, adding deionized water or anti-penetrate water; adding solvent into reaction kettle; adding bromine nitrogen isothiazole to be mixed and dissolved; adding bromine nitro compound; and adding C4H4CLNOS C4H5NOS to be mixed for 20-30mins.
Owner:TIANJIN CHEM RES & DESIGN INST
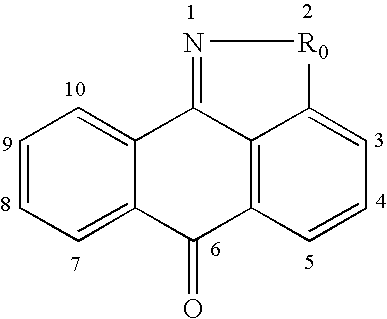
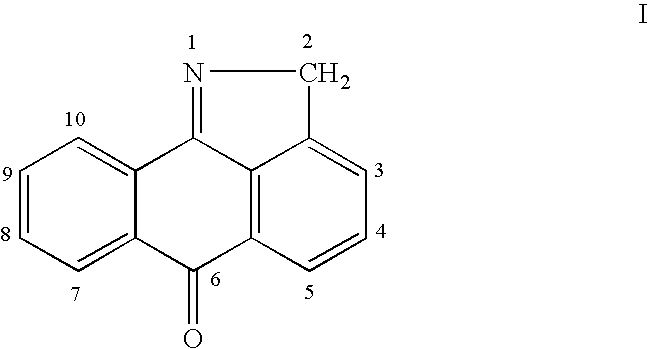
Methods for treating inflammatory conditions or inhibiting JNK
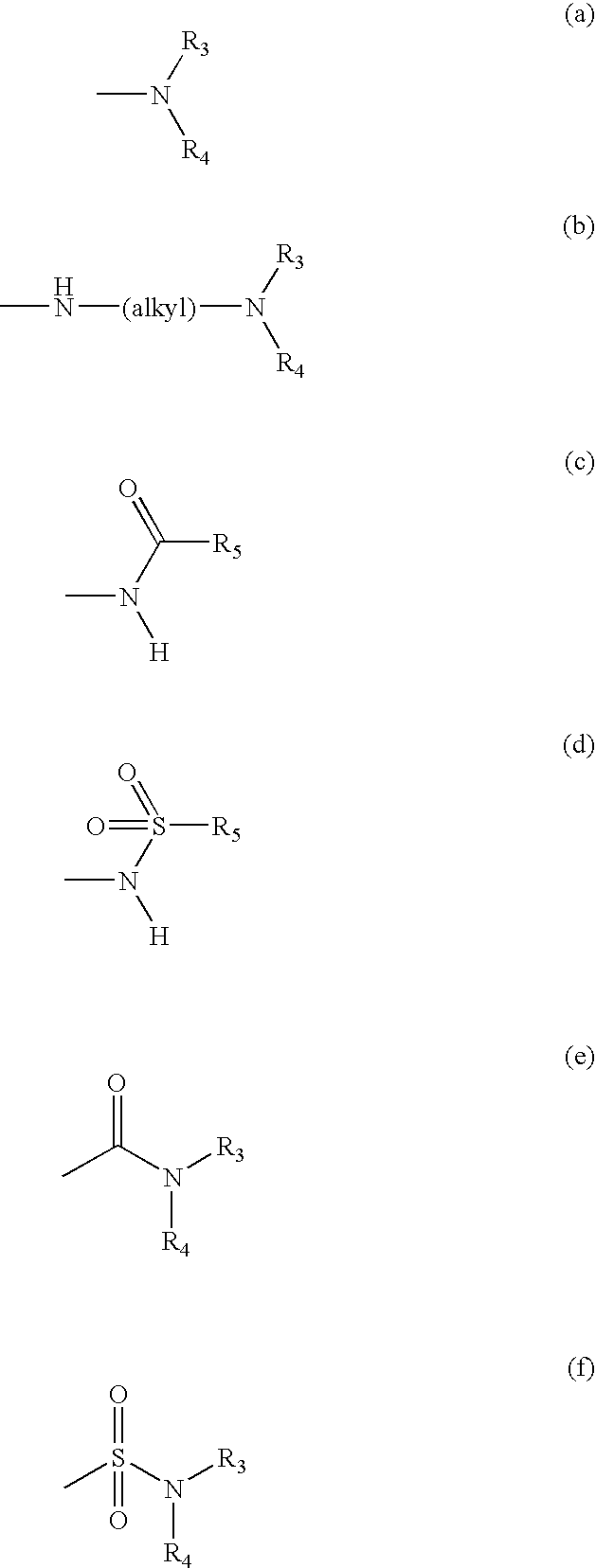
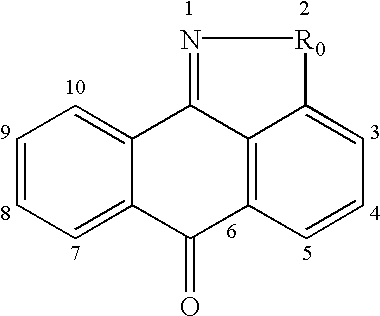
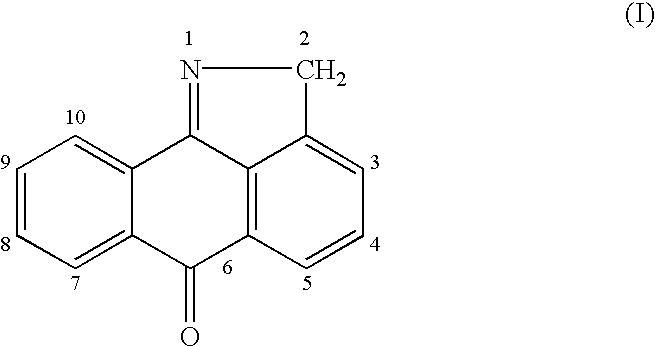
This invention is generally directed to methods for treating or preventing a disease or disorder comprising administering to a patient in need thereof an effective amount of a Jun N-terminal kinase (JNK) inhibitor, such as an isothiazoloanthrone, isoxazoloanthrone, isoindolanthrone, or derivative thereof having the general formula: and pharmaceutically acceptable salts thereof, wherein Ro is -CH2-, -SO-, -O-, -SO2-, or -S-.
Owner:SIGNAL PHARMA LLC
Isothiazoloanthrones, isoxazoloanthrones, isoindolanthrones and derivatives thereof as JNK inhibitors and compositions and methods related thereto
Isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof having the general formula: and pharmaceutically acceptable salts thereof, wherein R0 is -CH2-, -SO-, -O-, -SO2-, or -S-; compositions comprising the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof; and methods for treating or preventing a disorder alleviated by inhibiting Jun N-terminal kinase (JNK) by administering the isothiazoloanthrones, isooxazoloanthrones, isoindolanthrones, and derivatives thereof are described herein.
Owner:SIGNAL PHARMA LLC
Wood preservative compositions comprising isothiazolone-pyrethroids
InactiveUS20080175913A1High bactericidal activityHeavy metal active ingredientsOrganic active ingredientsChemistryPyrethrin I
The present invention relates to wood preservative compositions comprising an effective amount of a pyrethroid compound in combination with an isothiazolone compound, methods and processes for preserving wood using the wood preservative compositions of the invention, and wood comprising the wood preservative compositions of the invention.
Owner:OSMOSE
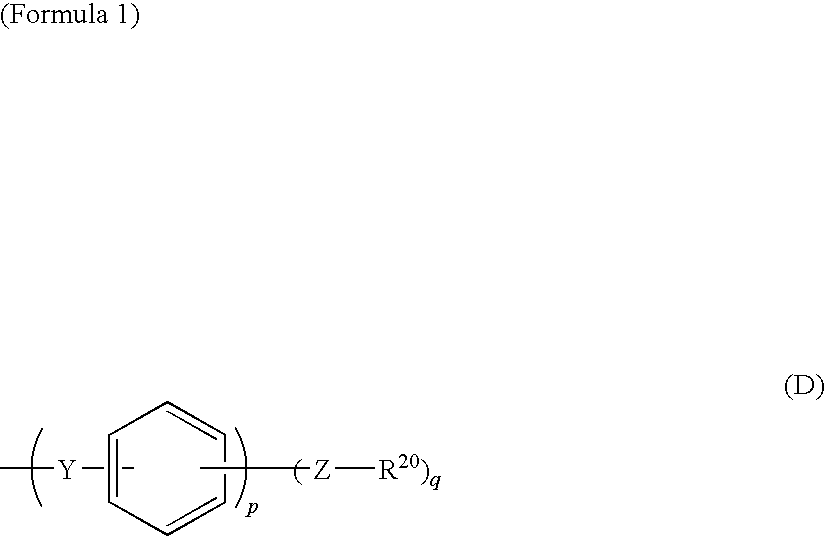
Electrode electrolyte for use in solid polymer fuel cell
ActiveUS20090130526A1Improve heat resistanceImprove proton conductivityActive material electrodesSolid electrolyte fuel cellsQuinoxalinePyridazine
This invention provides an electrode electrolyte for a solid polymer-type fuel cell, in which a cost problem and a problem related to recovery of catalyst metals are solved, having excellent proton conductivity, dimensional stability and heat resistance.An electrode electrolyte for a solid polymer electrolyte-type fuel cell contains a polymer, which has a polyphenylene structure as a main chain and both a sulfonic acid group and a nitrogen-containing heterocyclic group as a side chain. A side chain having the nitrogen-containing heterocyclic group has a structure represented by the following general formula (D).(In formula, Z represents at least one kind of structures selected from a group consisting of a direct bond, —O— and —S—, Y represents at least one kind of structures selected from a group consisting of —CO—, —SO2—, —SO—, —CONH—, —COO—, —(CF2)1— (1 is an integer of 1 to 10) and —C(CF3)2— and R20 represents a nitrogen-containing heterocyclic group. q represents an integer of 1 to 5 and p represents an integer of 0 to 4.)The above nitrogen-containing heterocyclic group is at least one kind of group derived from a compound selected from the group consisting of nitrogen-containing heterocyclic compounds including pyrrole, thiazole, isothiazole, oxazole, isoxazole, pyridine, imidazole, imidazoline, pyrazole, 1,3,5-triazine, pyrimidine, pyridazine, pyrazine, indole, quinoline, isoquinoline, purine, benzimidazole, benzoxazole, benzothiazole, tetrazole, tetrazine, triazole, carbazole, acridine, quinoxaline and quinazoline and derivatives thereof.
Owner:JSR CORPORATIOON +1
Preparation method of lurasidone
The invention provides a preparation method of lurasidone. On the basis of the existing preparation method of lurasidone, a one-pot method is adopted to replace the method including multiple steps and obtain a target product once. The preparation method comprises the following steps: adding 3-(1-piperazinyl)-1,2-benzisothiazole in toluene, stirring to dissolve; adding (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and an inorganic alkali, heating and carrying out reflux reaction for 12-36 hours; adding (3alpha R,4S,7R,7alpha S)4,7-methano-1H-isoindole-1,3(2H)-dione; heating and refluxing; recycling toluene at reduced pressure; adding ethyl acetate in the residue, stirring to dissolve, washing for 2-3 times with 5% hydrochloric acid, separating out the organic layers, drying for 20-120 minutes, filtering to remove the drying agent, concentrating the obtained ethyl acetate solution, dropwise adding concentrated hydrochloric acid, precipitating the solid, and performing suction filtration to obtain crude lurasidone; and refining crude lurasidone to obtain pure lurasidone. By adopting the preparation method of lurasidone, the solvent can be recycled conveniently and the method is simple in operation.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
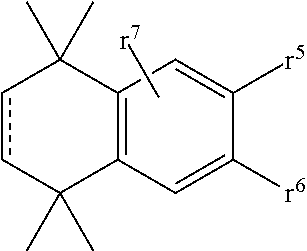
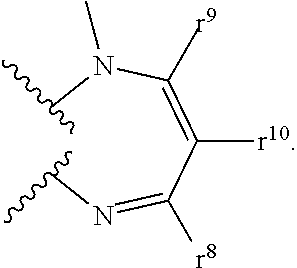
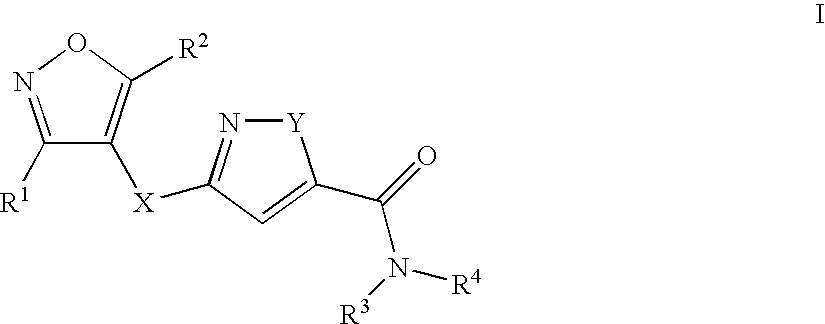
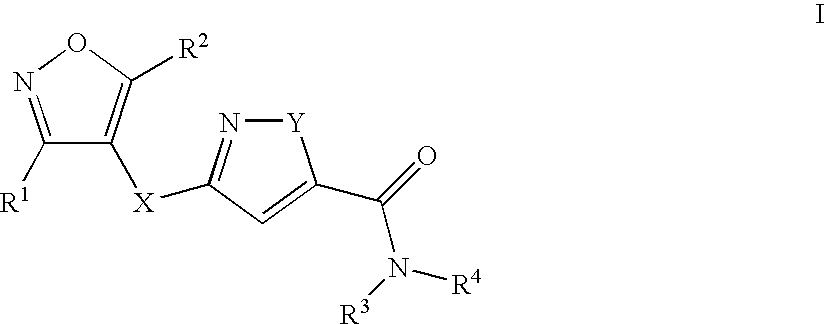
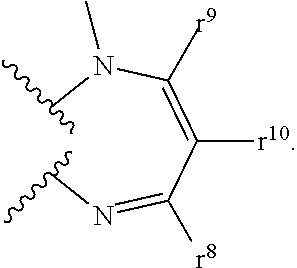
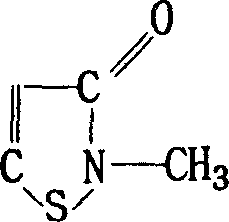
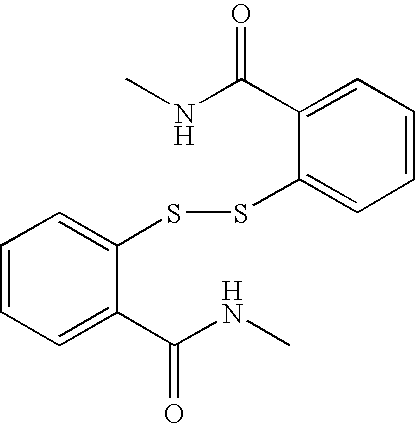
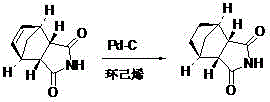
8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents
Owner:ACHILLION PHARMA INC
Hydrophilic antibacterial coating of aluminum-foil paper for cigarettes
ActiveCN103726402AImprove anti-mold and anti-bacterial propertiesImprove corrosion resistanceCoatings with pigmentsPaper/cardboardPolymer scienceOrganic dye
The invention discloses a hydrophilic antibacterial coating of aluminum-foil paper for cigarettes. The hydrophilic antibacterial coating is prepared from polyvinyl butyral (PVB) resin, polyacrylamide (PAM) resin, isothiazolone, a chitosan-copper complex, GR organic dye yellow, GR organic dye red, GR organic dye black, tap water, polyethylene wax as an antiwear agent, titanium dioxide, rosin and 95% ethanol. The coating is beneficial to the improvement of the quality and definition of a printed product, has good water resistance, is low in volatile substance content and strong in practicability, has excellent printing viscosity stability and low-combustion safety,is capable of improving the working environments of printing workers, simplifying the printing cleaning workload and increasing the production efficiency and can be popularized in food packaging and printing industries.
Owner:楚雄市华丽包装实业有限责任公司
Stabilized coating compositions containing isothiazolone
InactiveUS20030232906A1Enhanced nucleophilicityEasy to controlBiocideGroup 4/14 element organic compounds3-isothiazoloneCopper
High pH aqueous coating compositions containing 3-isothiazolone microbicide that have been stabilized against the degradation of the 3-isothiazolone by the addition of low levels of copper ion are disclosed. In particular, aqueous paint compositions containing pH-adjusting amine compounds, where the pH is above 9.5, are effectively stabilized when 1 to 200 ppm of copper ion is added to the paint composition before the 3-isothiazolone is combined with other paint components or if copper ion is added together with the 3-isothiazolone to the remaining paint components or if the 3-isothiazolone is added no more than 1 hour before copper ion is added to the composition.
Owner:GHOSH TIRTHANKAR
Preparation method of precious cream for deeply whitening and moistening skin
InactiveCN101791279AUnique long chain structureImprove antioxidant capacityCosmetic preparationsToilet preparationsEthylhexyl palmitateLycopene
The invention provides a preparation method of a precious cream for deeply whitening and moistening skin, which comprises the following steps: according to parts by weight, taking and adding 0.1-0.2 part of bisabolol into an oil tank, sequentially adding 1.3-1.8 parts of glyceryl stearate citrate, 0.5-0.7 part of beheneth-25, 3-5 parts of cetearyl alcohol, 2.0-2.2 parts of ethylhexyl palmitate, 2-4 parts of mineral oil, 0.8-1.2 parts of di-C12-13 alkyl malate and 2.5-3.2 parts of dimethyl polysiloxane, and heating to 80-88 DEG C; adding 73-78 parts of deionized water into a water tank, heating to 55-65 DEG C, adding 3-6 parts of glycerol and 0.2-0.5 part of allantoin, and heating to 80-88 DEG C; dissolving 0.3-0.7 part of lycopene by utilizing ethylhexyl palmitate for use; preheating and vacuumizing a main tank, sucking the materials in the oil tank and the water tank into the main tank, homogenizing for 7-9 minutes, adding 1.0-1.2 parts of polyacrylamide, and stirring for 2-3 minutes; when the temperature is reduced to 50 DEG C, adding the lycopene, and homogenizing for 2-3 minutes; when the temperature is reduced to 45 DEG C, adding 0.1-0.2 part of benzyl alcohol / chloromethyl isothiazolinone / methyl isothiazolinone, 1.5-2.2 parts of collagen, 0.02-0.04 part of rose essence and 0.5-1.2 parts of polyquaternium-51; and when the temperature is reduced to 40 DEG C and the pH value is 4.0-7.5, discharging.
Owner:WULUMUQI SHUIMOGOU SULIDAN FINE CHEM FACTORY
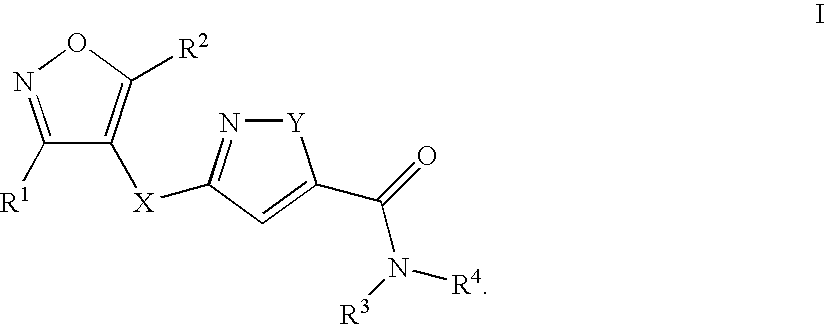
Isothiazole derivatives as GPR120 agonists for the treatment of type II diabetes
Disclosed are compounds, compositions and methods for treating of disorders that are affected by the modulation of the GPR120 receptor. Such compounds are represented by Formula (I) as follows:wherein R1, G, and Q are defined herein.
Owner:JANSSEN PHARMA NV
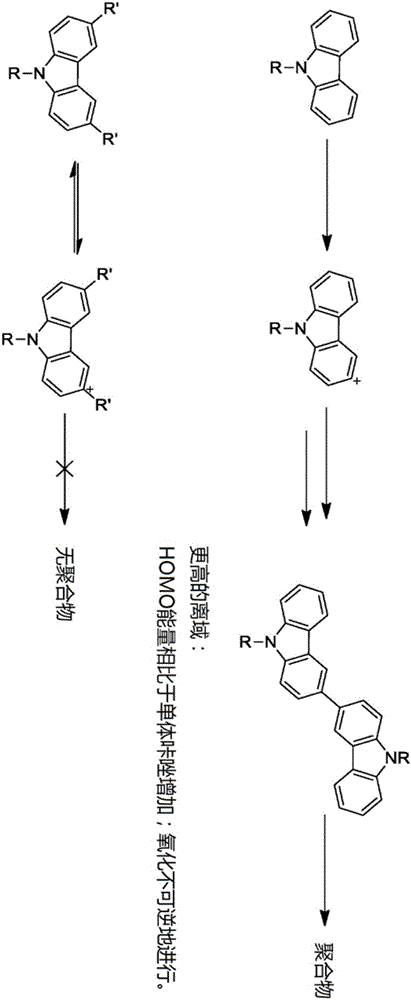
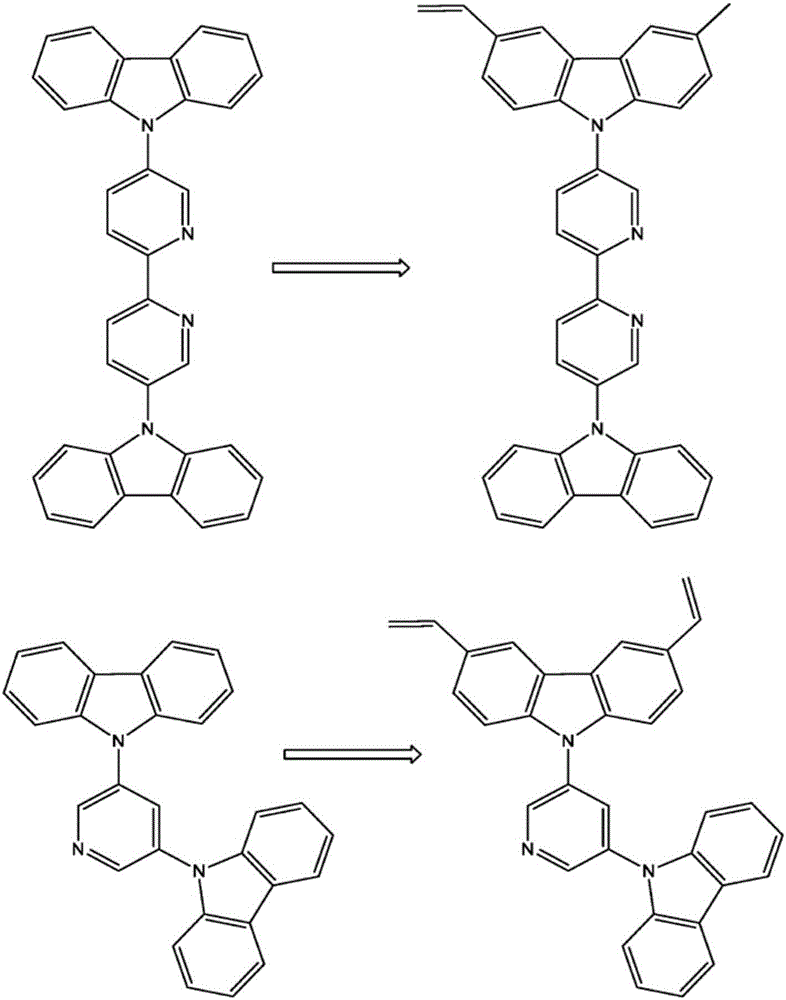
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
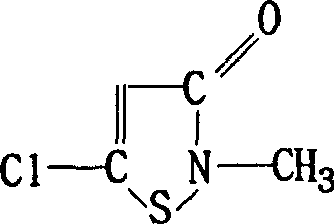
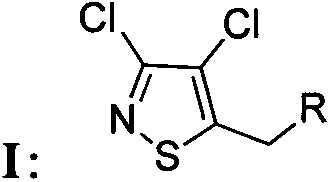
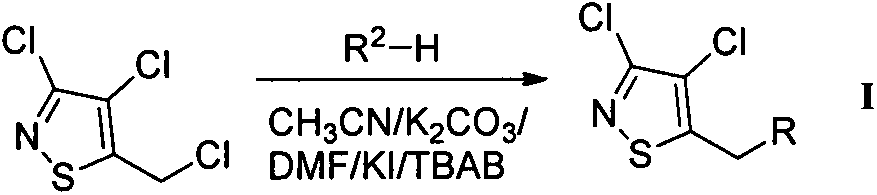
Chloroisothiazole neonicotine compound, as well as preparation method and application thereof
The invention provides a chloroisothiazole neonicotine compound, as well as a preparation method and application thereof. The 3,4-dichloroisothiazole neonicotine compound has a chemical structural general formula as shown in a formula I. The formula is as shown in the specification. The invention further discloses a structure general formula, a synthesizing method of the compound, application of the compound as insecticides, bactericides and plant virucides, application of a composition of the compound, agriculturally acceptable auxiliary or synergist and commercial insecticide, bactericide, plant virucdies and acaricides in prevention and control of insect pests, diseases and virus diseases of agricultural, forestal and horticultural plants and a preparation method of the composition.
Owner:NANKAI UNIV
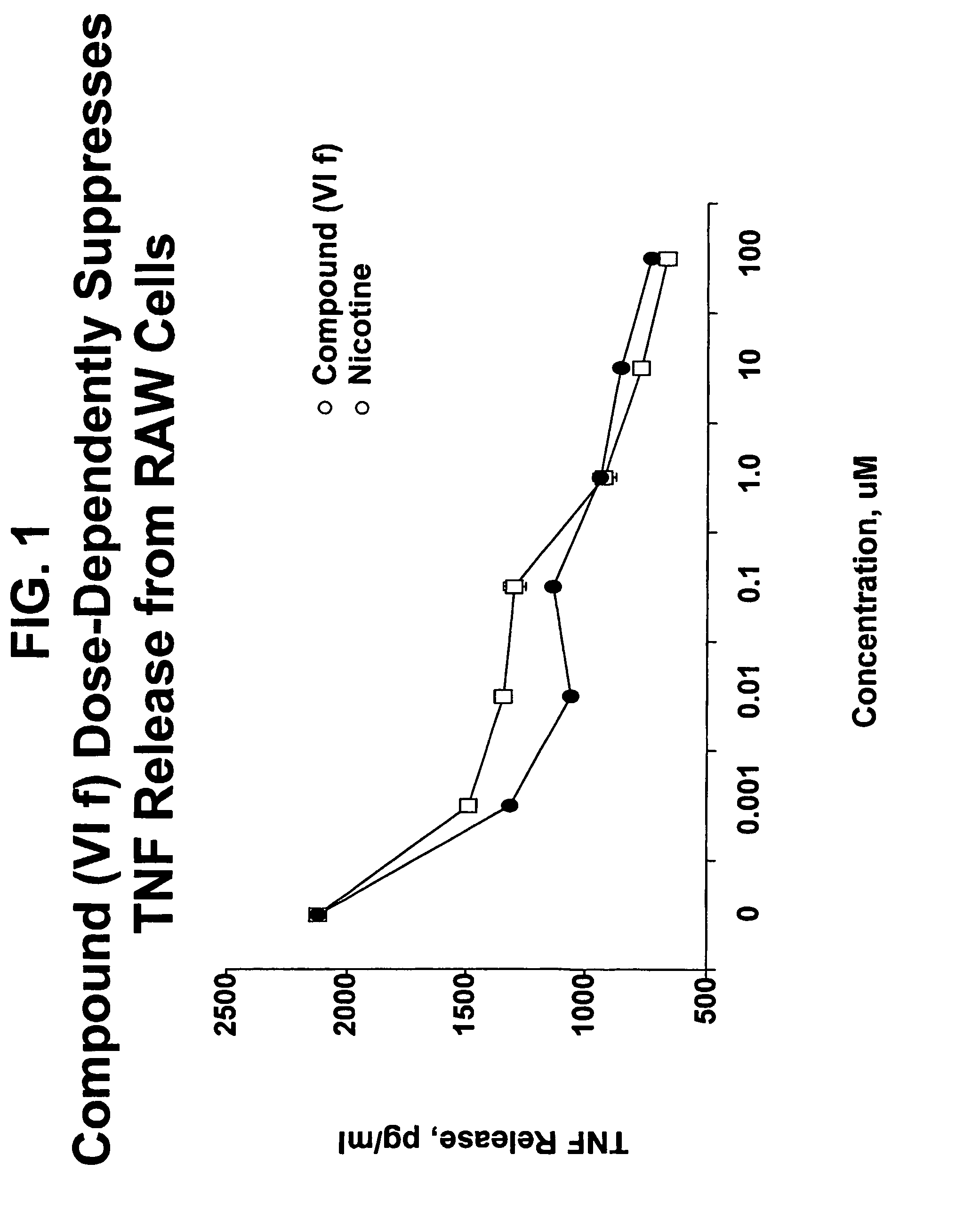
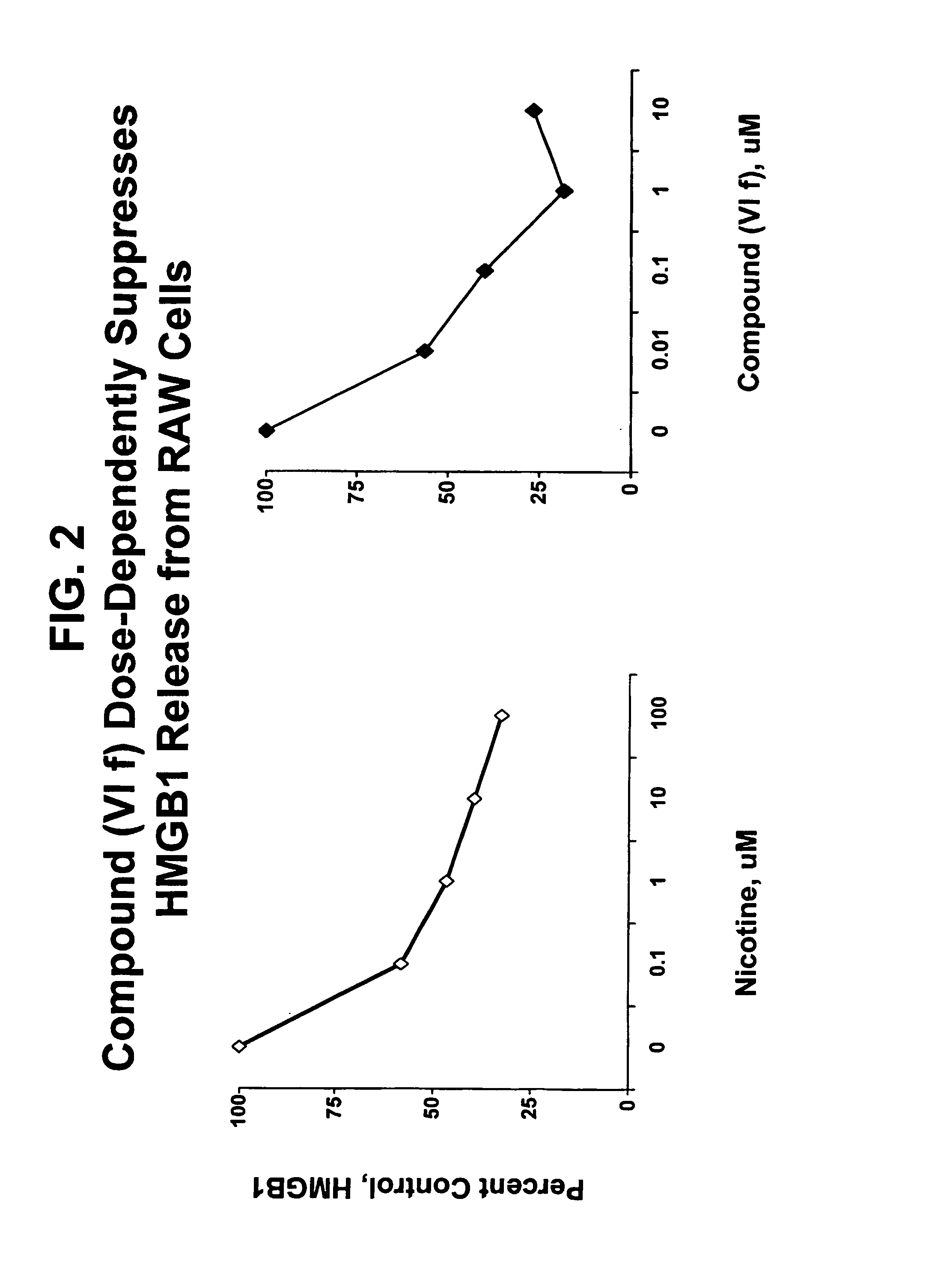
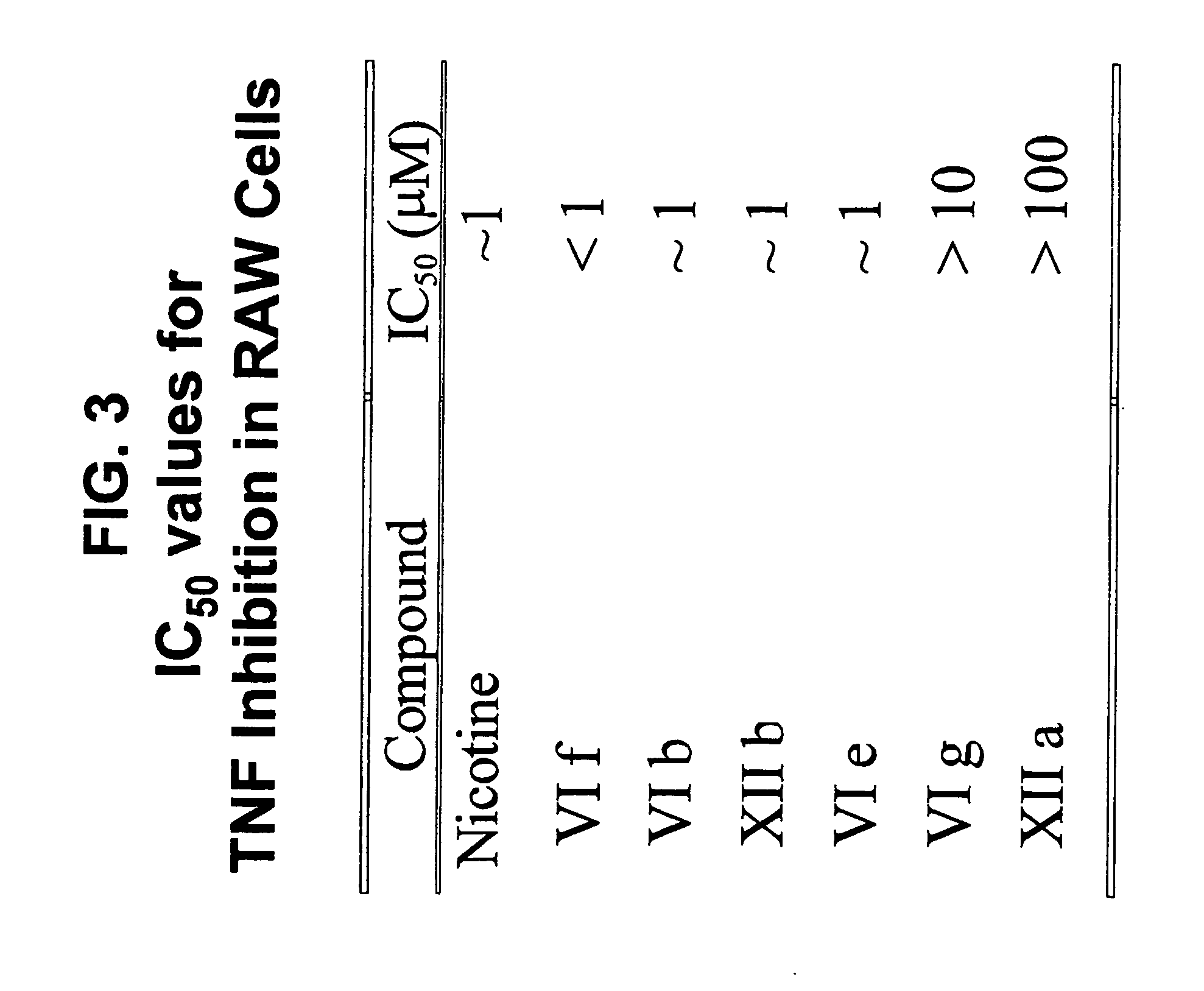
Isoxazole and isothiazole compounds useful in the treatment of inflammation
Owner:THE FEINSTEIN INST FOR MEDICAL RES
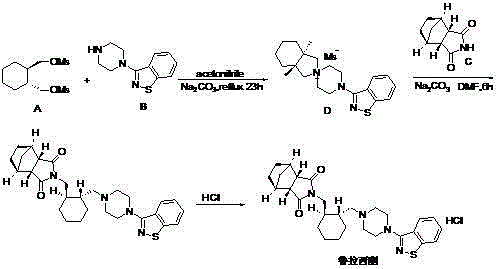
Preparation method of ziprasidone
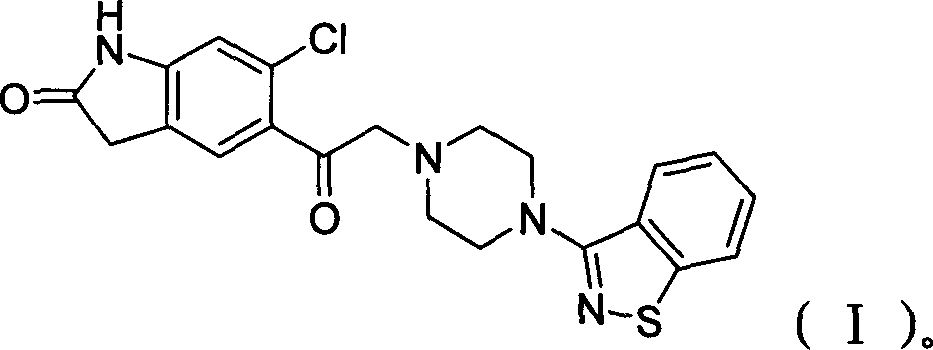
The invention discloses a making method of qilaxi ketone with structural formula as formula (I), which is characterized by the following: the chemical name of compound is 5-(2-(4-(1,2-benzo isothiazole-3-base)-piperazine) acetyl)-6-chloride-1, 3-dihydrogen-2H-indole-2-ketone, which is reduced in the organic acid solvent; the solvent is C1-C6 paraffin acid substituted by at least one halogenate atom with carboxyl function group.
Owner:ZHEJIANG MENOVO PHARMA
Wood preservative formulations comprising isothiazolones which provide protectin against surface staining
The present invention provides a wood preservative formulation comprising an isothiazolone, an organic fungicidal timber decay preservative and an unsaturated carboxylic or sulphonic acid, salt or precursor thereof. The formulations of the invention are surprisingly effective at protecting wood and other cellulosic substrates, in particular at providing prolonged protection against in- service surface staining. The invention also provides methods for treating wood and other cellulosic substrates with said formulations.
Owner:ARCH TIMBER PROTECTION
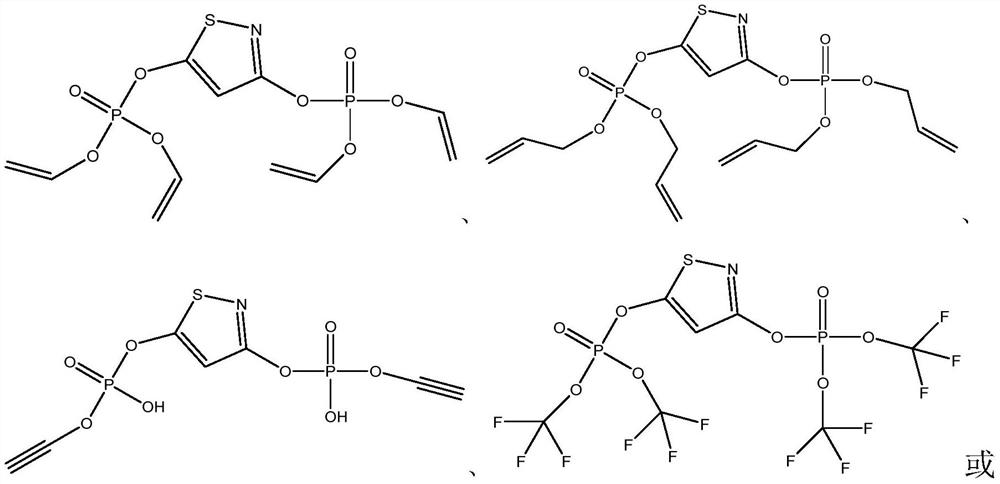
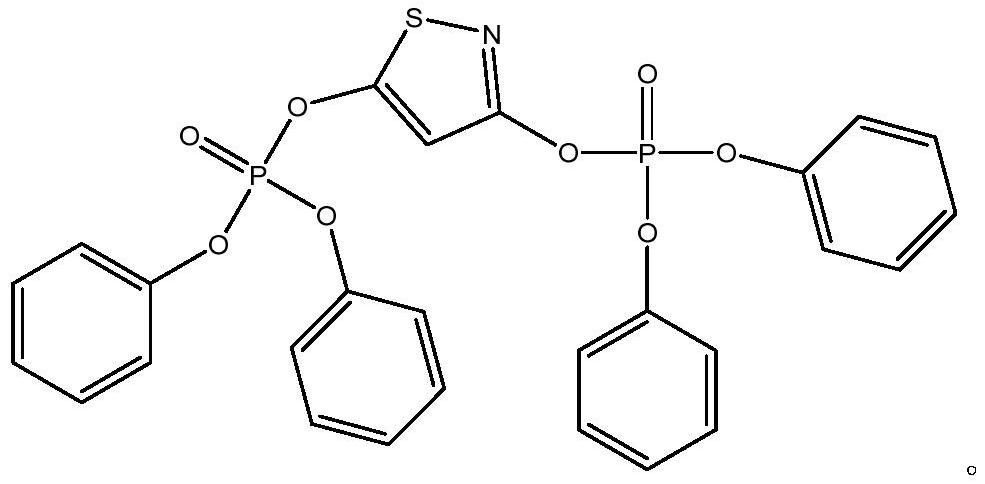
Electrolyte additive for improving battery high-temperature gas expansion, electrolyte and lithium ion battery containing electrolyte
ActiveCN112838270AImprove cycle performanceImprove stabilitySecondary cells servicing/maintenanceOrganic electrolytesElectrolytic agentThiazole
The invention discloses an electrolyte additive for improving high-temperature flatulence of a battery, and relates to the technical field of lithium ion batteries, the additive comprises a 1,3-diphosphate-isothiazole compound and a water removal additive. The structural general formula of the 1,3-diphosphate-isothiazole compound is shown in the specification, wherein R1 and R2 are respectively and independently selected from one of H, C1-8 alkyl, C4-10 cycloalkyl, C2-10 alkenyl, C2-10 alkynyl, C6-16 aryl, C6-16 heteroaryl and part of fluoro or perfluoro compounds of the H, the C1-8 alkyl, the C4-10 cycloalkyl, the C2-10 alkenyl, the C2-10 alkynyl, the C6-16 heteroaryl and the part of fluoro or perfluoro compounds of the C6-16 heteroaryl. The invention also provides an electrolyte containing the additive and a lithium ion battery. The electrolyte has the beneficial effects that the special high-temperature gas expansion improving additive is added into the electrolyte, so that the reaction between the electrolyte and positive and negative electrode materials in the lithium ion battery under a high-temperature condition is inhibited, the stability of the positive and negative electrode materials under a high-temperature environment is improved, and the storage gas expansion and cycle performance of the battery under the high-temperature condition is improved.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Method for enhancing alkali resistance and oxidation resistance of benzoisothiazole disperse dyes
ActiveCN111995879AIncrease brightnessBright colorMonoazo dyesDyeing processDisperse dyeTextile technology
The invention discloses a method for enhancing alkali resistance and oxidation resistance of benzoisothiazole disperse dyes, and belongs to the technical field of textiles. The method starts from thestructural design of the dye; an azo alkali-resistant disperse dye taking the benzoisothiazole as a diazo component is synthesized; different groups are introduced into the coupling component to enhance the alkali resistance and oxygen bleaching resistance of the heterocyclic azo disperse dye, so that a series of benzoisothiazole disperse dyes with alkali resistance and oxidation resistance strength difference gradients are obtained; the disperse dye capable of meeting the requirements of a polyester-cotton blended fabric bleached cotton and disperse dyeing polyester one-bath process or a polyester fabric alkali deweighting and disperse dyeing one-bath process is determined, and a reference can be provided for the structural design of alkali-resistant and oxidation-resistant disperse dyes.
Owner:QINGDAO UNIV +1
Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone)
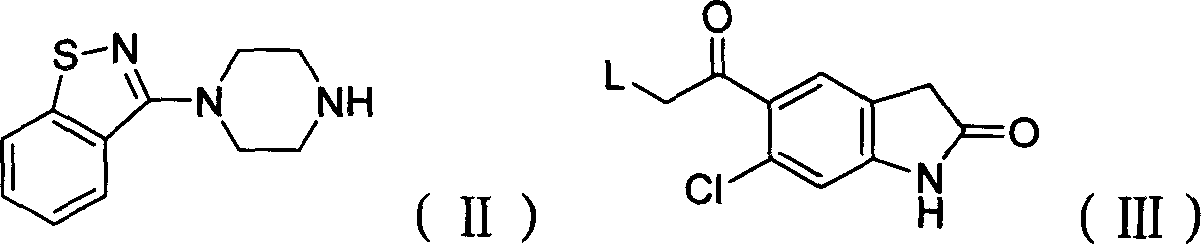
The present invention provides a novel, industrially easily realisable and economically preferable process for production of pure 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one i.e., ziprasidone hydrochloride shown in the reaction scheme (II), (III), (IV), (V) and (VI). According to the invention the intermediate compound 5-(2-bromoethyl)-6-chloro-1,3-dihydro-2H-indol-2-one of Formula (III) is produced from 5-(2-bromoacethyl)-6-chloro-1,3-dihydro-2H-indole-2-one of Formula (IV). The highly pure ziprasidone base of Formula (II) is obtained in the reaction of 3-piperazinyl-1,2-benzisothiazol of Formula (VI) with 5-(2-bromoethyl)-6-chloro-1,3-dihydro-2H-indol-2-one of Formula (III) in an organic solvent or organic solvent mixture.
Owner:RICHTER GEDEON NYRT
Low-melting biocidal formulation
ActiveUS20090258916A1Prevent rapid leachingWide variationBiocideDead animal preservationChemical compositionRoom temperature
A low-melting biocidal composition comprising 4,5-dichloro-2-octyl-3(2H)-isothiazolone, 3-iodopropargyl-N-butyl carbamate and at least one C1-C4 alkyl 4-hydroxybenzoate. The composition is stable with regard to agglomeration and crystallization at room temperature.
Owner:ROHM & HAAS CO
Benzoisothiazole compound, preparation and uses and disperse dyes composition
InactiveCN1995026AImprove dyeing effectHigh color fastnessMonoazo dyesOrganic chemistryDisperse dyeMicroparticle
The invention discloses a benzo-isothiazole compound and making method and application with structure as formula (I) as well as dye composition with benzo-isothiazole compound, which can make dispersing blue dye through microparticle process, spraying and drying.
Owner:ZHEJIANG LONGSHENG GROUP
Heterocyclic compound
InactiveUS9156837B2Enhanced inhibitory effectAgent for prophylaxis and treatmentNervous disorderOrganic chemistryThiazolePyrazole
A heterocyclic compound having an RORγt inhibitory activity, which is a compound of formula (I) or a salt thereof is provided. The compound has ring A, which is an optionallysubstituted cyclic group and is bound to a pyrazole ring though Q. Q is a bond, optionally substituted C1-10 alkylene, optionally substituted C2-10 alkenylene, or optionally substituted C2-10 alkynylene. R1 is a substituent. Ring B is a thiazole ring, an isothiazole ring or a dihydrothiazole ring, each of which is optionally further substituted by a substituent in addition to R2. R2 is an optionally substituted cyclyl-carbonyl-C1-6 alkyl group, an optionally substituted aminocarbonyl-C1-6 alkyl group, an optionally substituted cyclyl-C1-6 alkyl group, an optionally substituted cyclyl-C1-6 alkylamino-carbonyl group, an optionally substituted aminocarbonyl-C2-6 alkenyl group, an optionally substituted C1-6 alkylcarbonylamino-C1-6 alkyl group, an optionally substituted cyclyl-aminocarbonyl group, an optionally substituted cyclyl-carbonyl group or an optionally substituted non-aromatic heterocyclic group.
Owner:TAKEDA PHARMA CO LTD
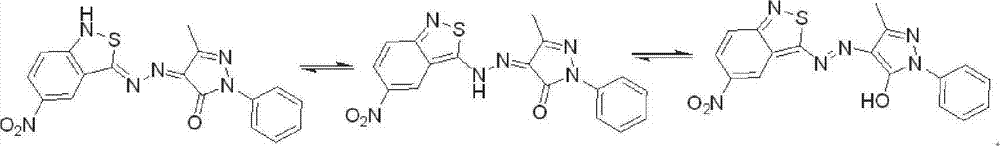
Benzo isothiazole azo pyrazolone disperse dye as well as preparation method and use thereof
InactiveCN102924960AEasy to identifyAccurate identificationMonoazo dyesColor/spectral properties measurementsDisperse dyeHeteroatom
The invention discloses a benzo isothiazole azo pyrazolone disperse dye. The benzo isothiazole azo pyrazolone disperse dye is a compound having the structure shown in a formula (I) in the specification. Conjugate migration of hydrogen connected with a heteroatom in the benzo isothiazole azo pyrazolone heterocyclic ring disperse dyes is performed among benzo isothiazole ring, azo and pyrazolone to display bright-colored yellow, red and blue; and moreover, the dye is large in hue span and rich in colors; and therefore, the benzo isothiazole azo pyrazolone disperse dye can be used for preparing a fiber identification coloring agent. The invention discloses a preparation method of the benzo isothiazole azo pyrazolone disperse dye.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
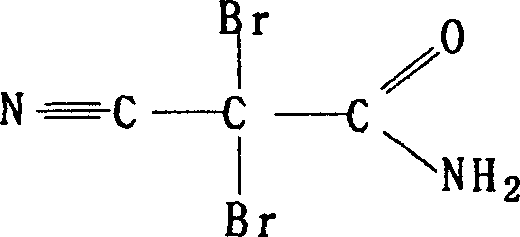
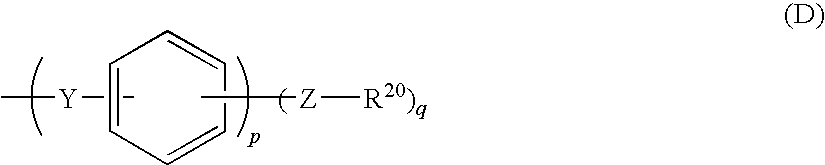
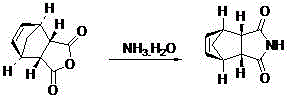
![8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents 8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents](https://images-eureka.patsnap.com/patent_img/5a568bcc-1f27-474b-9176-2d1419f17c5e/US07199128-20070403-C00001.png)
![8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents 8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents](https://images-eureka.patsnap.com/patent_img/5a568bcc-1f27-474b-9176-2d1419f17c5e/US07199128-20070403-C00002.png)
![8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents 8-N-substituted-2H-isothiazolo[5,4-b]quinolizine-3,4-diones and related compounds as antiinfective agents](https://images-eureka.patsnap.com/patent_img/5a568bcc-1f27-474b-9176-2d1419f17c5e/US07199128-20070403-C00003.png)
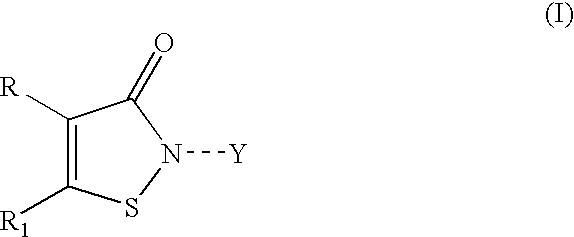
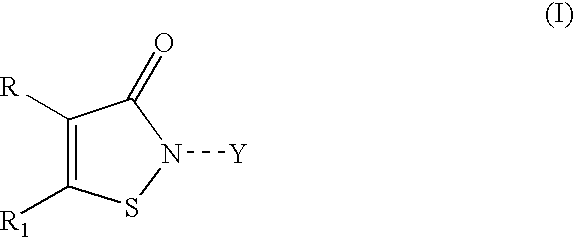
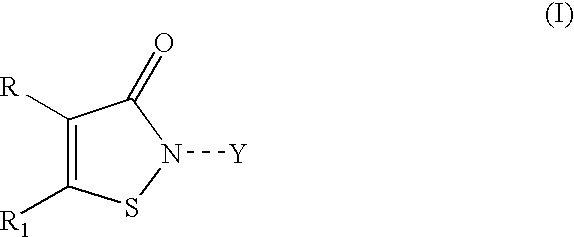


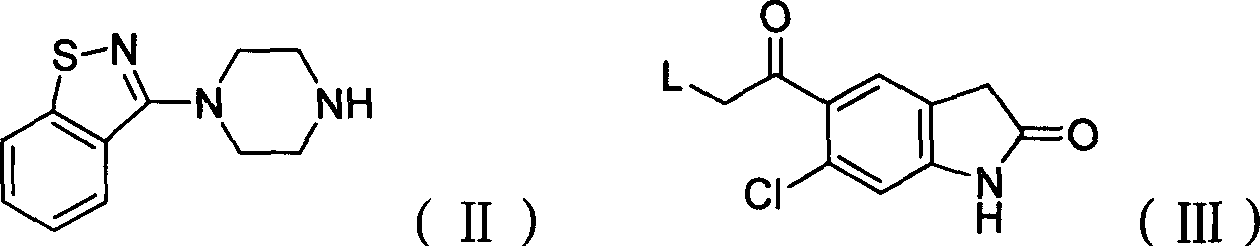
![Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone) Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone)](https://images-eureka.patsnap.com/patent_img/9e22b7f8-6870-4b47-b63d-41f00bc05186/a200780015835e00101.PNG)
![Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone) Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone)](https://images-eureka.patsnap.com/patent_img/9e22b7f8-6870-4b47-b63d-41f00bc05186/a200780015835c00021.PNG)
![Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone) Novel process for production of 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2h-indol-2-one (ziprasidone)](https://images-eureka.patsnap.com/patent_img/9e22b7f8-6870-4b47-b63d-41f00bc05186/a200780015835d00031.PNG)