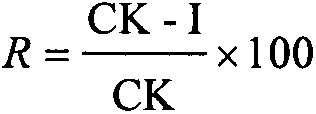Chloroisothiazole neonicotine compound, as well as preparation method and application thereof
A neonicotinoid and isothiazole technology, applied in the field of chloroisothiazole neonicotinoid heterocyclic compounds, can solve unscientific and reasonable problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
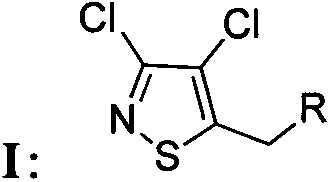
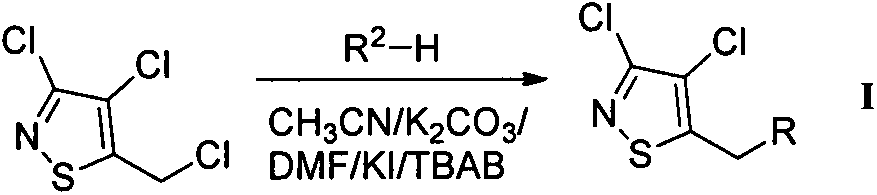
[0029] Preparation of chloroisothiazole neonicotinoid compound I:
[0030] Into a 100-mL round-bottomed flask were sequentially added 3,4-dichloro-5-chloromethylisothiazole (0.3 g, 1.45 mmol), the anonicotinoid intermediate R 2-H (2.03 mmol), potassium carbonate (0.28 g, 2.03 mmol), potassium iodide (33.2 mg, 0.20 mmol), and the phase transfer catalyst tetrabutylammonium bromide (TBAB) (32.2 mg, 0.10 mmol), Then add 30 milliliters of acetonitrile solution, finally add 1 milliliter of N,N-dimethylformamide (DMF), stir and reflux at 80 degrees Celsius for 3 to 5 hours, TLC detects the reaction, after the reaction is completed, remove the insolubles by filtration, the filtrate Concentrate under reduced pressure, add ethyl acetate to the residue, wash once with saturated sodium bicarbonate solution and saturated sodium chloride solution respectively, keep the organic phase, back-extract the aqueous phase with ethyl acetate twice, combine the organic phases, Dry over magnesium sul...
Embodiment 2
[0032] The antibacterial activity assay result of chloroisothiazole neonicotinoid compound I of the present invention:
[0033] The code name and title of the common phytopathogenic fungi tested by the present invention are as follows: AS: tomato early blight fungus, its Latin name: Alternariasolani, BC: cucumber gray mold, its Latin name: Botrytiscinerea, CA: peanut brown spot fungus, its Latin name: Cercospora arachidicola, GZ: wheat scab, its Latin name: Gibberellazeae, PI: Potato infestans, its Latin name: Phytophthorainfestans (Mont.) deBary, PP: Apple ringworm, its Latin name : Physalosporapiricola, PS: Rice sheath blight, its Latin name is: Pelliculariasasakii, RC: Rhizoctonia graminearum, its Latin name is: Rhizoctoniacerealis, SS: Sclerotinia sclerotiorum, its Latin name is: Sclerotiasclerotiorum, these strains It is very representative and can represent most of the pathogenic bacteria species that occur in the field in agricultural production.
[0034] Compound zgn0...
Embodiment 3
[0037] Chlorinated isothiazole neonicotinoid compound I insecticidal activity of the present invention:
[0038] The insecticidal activity assay result of chloroisothiazole neonicotinoid compound I is shown in Table 4, and it can be seen in table 4: most of chloroisothiazole neonicotinoid compound I of the present invention have better insecticidal activity, and in the test When the concentration of the agent is 100 mg / L, after 72 hours of observation, the chloroisothiazole neonicotinoid compound I of the present invention has a good poisoning effect on aphids, and the insecticidal activity of the compound zgn07-40-1 is 92.23 %, higher than 51.83% of the commercial variety imidacloprid with the closest chemical structure and more than 40%; the insecticidal activity of compound zgn08-4 is 57.32%, higher than 39.88% of the commercial variety thiamethoxam with the closest chemical structure % is more than 10%; the inhibitory activity of compound zgn09-114 is 50.55%, which is more...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com