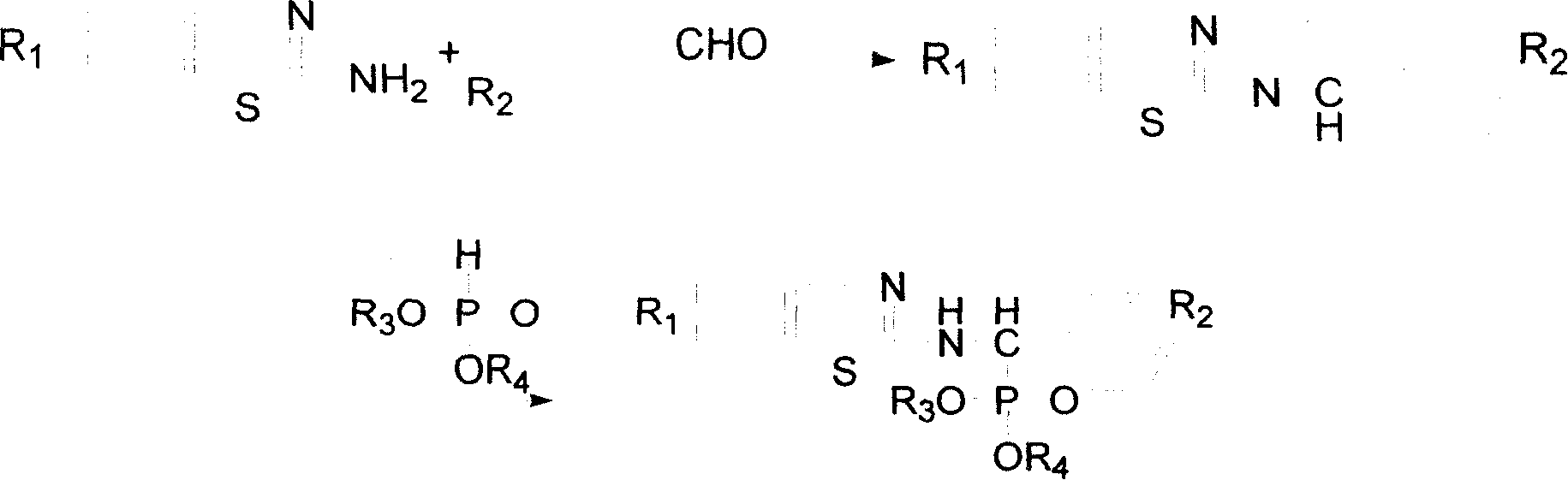
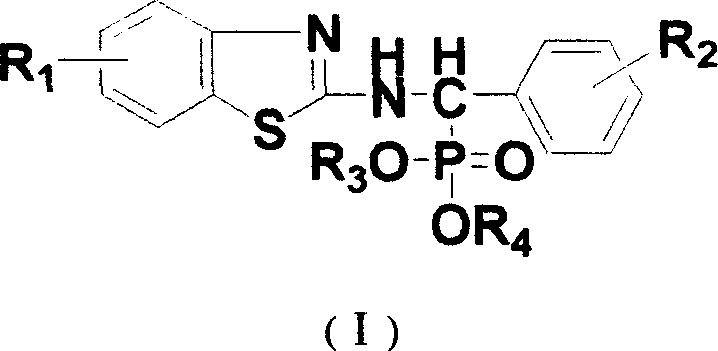
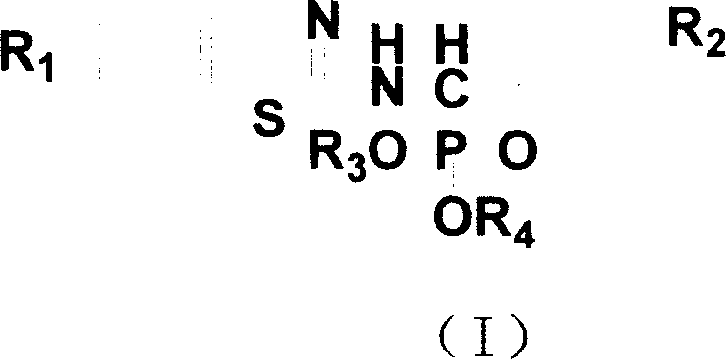N-substituted benzothiazolyl-1-substituted phenyl-0, 0-dialkyl-alpha-amino phosphonate ester derivatives preparation and application
An aminophosphonate and substituent technology, applied in the fields of N-substituted benzothiazolyl-1-substituted phenyl-O,O-dialkyl-α-aminophosphonate derivatives and their preparation and use , which can solve the problems of low product yield, reduced amine nucleophilicity, and failure to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, the synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)-O, O-dimethyl-α-aminophosphonate (compound number for a)
[0032] (1) Synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)imine
[0033] Put 2-amino-6-methoxybenzothiazole (4mmol), 4-fluorobenzaldehyde (4mmol) and 15mL toluene into a 25mL three-necked round-bottomed flask with a water trap device, heat to reflux after stirring at room temperature, and Boiling dehydration, followed by TLC (petroleum ether: ethyl acetate = 2:1 volume ratio), the reaction raw material point disappeared after about 1-2h, stop the reaction, directly go to the next step reaction, the resulting solution is N-(6-formazan Oxybenzothiazol-2-yl)-1-(4-fluorophenyl)imine solution. After purification, light yellow crystals were obtained with a yield of 82.4%, m.p.110-112°C, 1 H NMRδ: 3.70(s, 3H, OCH 3 ), 4.05-4.10 (m, 1H, CH), 7.01-7.53 (m, 7H, Ar-H).
[0034] Using the same synthesis method, different sol...
Embodiment 2
[0037] Embodiment two, the synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)-O, O-diethyl-α-aminophosphonate (compound number for b)
[0038] (1) Synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)imine was synthesized according to the method and conditions of Example 1 (1).
[0039] (2) Synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)-O, O-diethyl-α-aminophosphonate
[0040] Synthesized according to the method and conditions of Example 1 (2), only dimethyl phosphite was replaced by diethyl phosphite, the yield was 72.3%, m.p.198-200°C.
Embodiment 3
[0041] Embodiment three, the synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)-O, O-di-n-propyl-α-aminophosphonate (compound number c)
[0042] (1) Synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)imine
[0043] Synthesize as in Example 1 (1) method and conditions.
[0044] (2) Synthesis of N-(6-methoxybenzothiazol-2-yl)-1-(4-fluorophenyl)-O, O-di-n-propyl-α-aminophosphonate
[0045] Synthesized according to the method and conditions of Example 1 (2), only replacing dimethyl phosphite with di-n-propyl phosphite, yield 71.6%, m.p.130-131.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com