Fungicide composition
A technology of fungicides and compositions, applied in the field of agricultural fungicides, can solve the problems of no reports of multi-active component compositions, no reports of in-depth research on compounds, etc., to delay drug resistance and systemic activity of pathogens Excellent, good synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1. Synthesis of intermediate (E)-1-(3-trifluoromethylphenyl)ethanone oxime
[0036]
[0037] Dissolve 1.9 g of m-trifluoromethyl acetophenone in ethanol, then add 1 g of hydroxylamine hydrochloride (dissolved in 2 ml of water), stir at room temperature, then add 0.6 g of sodium hydroxide (dissolved in 2 ml of water), and reflux reaction 2- After 4 hours, TLC detected that the reaction was complete, evaporated part of the solvent under reduced pressure, poured the reaction solution into ice water, added acetic acid to adjust the pH to about 6, precipitated a solid, filtered, washed with water, and rinsed with a small amount of ethanol to obtain the product.
example 2
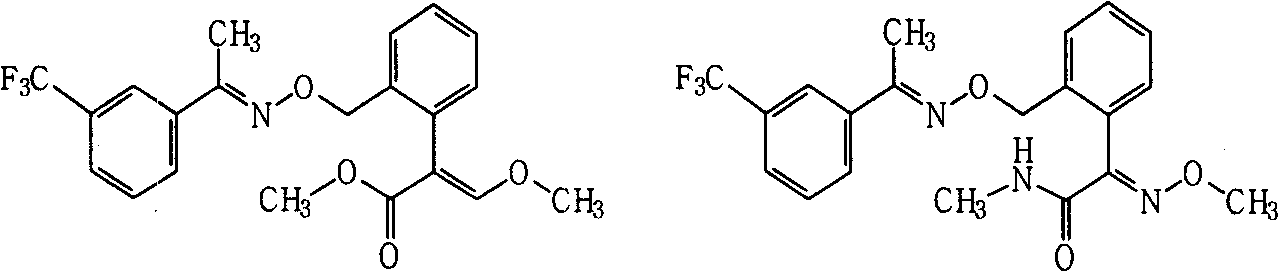
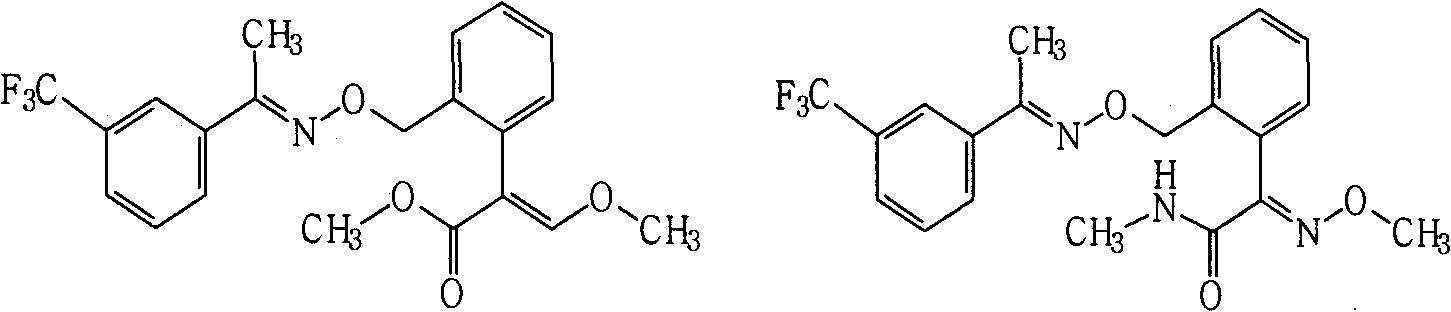
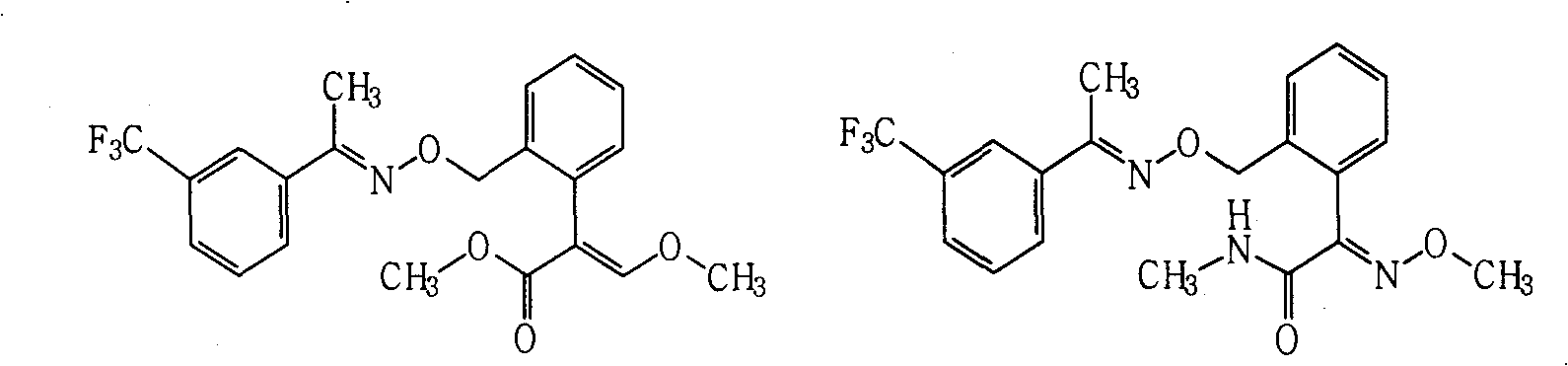
[0038] Example 2. Compound A 1 Synthesis
[0039]
[0040] Dissolve 0.2 g of (E)-1-(3-trifluoromethylphenyl) acetoxime in 5 ml of N,N-dimethylformamide, add 0.10 g of 60% sodium hydride washed with petroleum ether, Stir for 0.5h. Add (E)-2-[2-(bromomethyl)phenyl]-3-methoxymethyl acrylate (prepared by known methods, see US4723034 and US5554578, etc.) 0.3g, and the reaction temperature is 40°C , stirred for 3h. After the reaction was monitored by TLC, the reaction solution was poured into 50 ml of saturated brine, extracted three times with 100 ml of ethyl acetate, and dried. After precipitation, it was purified by column chromatography (eluent: ethyl acetate and petroleum ether (boiling range: 60°C-90°C), volume ratio: 1:4, the same below) to obtain 0.18 g of viscous product.
[0041] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0042] δppm 2.26(3H, s), 3.69(3H, s), 3.82(3H, s), 5.17(2H, s), 7.18(1H, m), 7.34(2H, m), 7.50(2H, m), 7....
example 3
[0043] Example 3. Compound A 2 Synthesis
[0044]
[0045] Dissolve 0.2g trifloxystrobin in 5ml tetrahydrofuran, add dropwise a little excess of 25-30% methylamine aqueous solution, and then heat to reflux for 1h, after the reaction is monitored by TLC, desolventize, add water, and extract three times with 30ml ethyl acetate ,dry. After precipitation, the product was purified by column chromatography to obtain 0.16 g of an oily product.
[0046] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0047] δppm 2.22(3H, s), 2.95(3H, d), 3.95(3H, s), 5.14(2H, s), 6.75(1H, m), 7.20(1H, m), 7.37-7.52(4H, m ), 7.60 (1H, m), 7.79 (1H, m), 7.89 (1H, m).
[0048] Formulation example of the composition
[0049] The addition amount of each component is measured in parts by weight, wherein the active substance is the A and B components of the present invention and mixed and added according to the stated ratio.
[0050] Example 1. Preparation of 20% EC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com