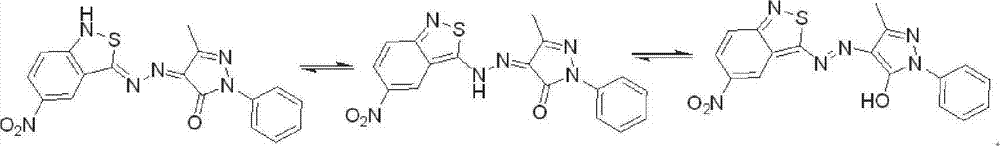
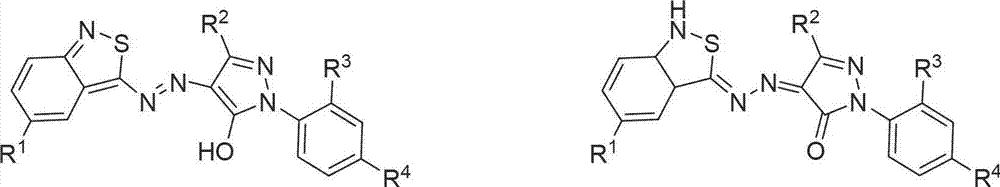Benzo isothiazole azo pyrazolone disperse dye as well as preparation method and use thereof
A technology of benzisothiazopyrazolone and disperse dyes, which is applied in azo dyes, monoazo dyes, dyeing methods, etc., and can solve the problems of counterfeit and inferior products, complex components, and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Weigh 0.227g (3.3mmol) NaNO 2 , add 2.5ml (39.6mmol, 1:12) concentrated sulfuric acid, stir quickly, when heated to 65-70°C (the temperature should not exceed 80°C), stop heating, and cool down naturally, if there is salt precipitation, heat it slightly to dissolve it , to obtain nitrosylsulfuric acid. Weigh 0.585g (3mmol) of 3-amino-5-nitro-2,1-benzisothiazole, dissolve it in the above solution, and then add 2ml of concentrated sulfuric acid to obtain a dark brown solution in which all solids are dissolved.
[0058] Take 5ml of concentrated sulfuric acid and 7.5ml of glacial acetic acid, mix it into a 40% sulfuric acid / acetic acid solution in a 250ml three-necked bottle, add a little ice cubes, and put it in an ice-salt bath. Add the above-prepared dark brown solution dropwise into the sulfuric acid solution, and stir rapidly. After the dropwise addition, react for 10-15 minutes.
[0059] Weigh 0.523g (3mmol) 1-phenyl-3-methyl-5-pyrazolone, put it into a 250ml three...
Embodiment 2
[0063] Weigh 0.227g (3.3mmol) NaNO 2 , add 2.5ml (39.6mmol, 1:12) concentrated sulfuric acid, stir quickly, when heated to 65-70°C (the temperature should not exceed 80°C), stop heating, and cool down naturally, if there is salt precipitation, heat it slightly to dissolve it , to obtain nitrosylsulfuric acid. Weigh 0.585g (3mmol) of 3-amino-5-nitro-2,1-benzisothiazole, dissolve it in the above solution, and then add 2ml of concentrated sulfuric acid to obtain a dark brown solution in which all solids are dissolved.
[0064] Take 5ml of concentrated sulfuric acid and 7.5ml of glacial acetic acid, mix it into a 40% sulfuric acid / acetic acid solution in a 250ml three-necked bottle, add a little ice cubes, and put it in an ice-salt bath. Add the above-prepared dark brown solution dropwise into the sulfuric acid solution, and stir rapidly. After the dropwise addition, react for 10-15 minutes.
[0065] Weigh 3mmol 1-phenyl-3-methoxycarbonyl-5-pyrazolone, put it into a 250ml three...
Embodiment 3
[0068] Weigh 0.227g (3.3mmol) NaNO 2, add 2.5ml (39.6mmol, 1:12) concentrated sulfuric acid, stir quickly, when heated to 65-70°C (the temperature should not exceed 80°C), stop heating, and cool down naturally, if there is salt precipitation, heat it slightly to dissolve it , to obtain nitrosylsulfuric acid. Weigh 0.585g (3mmol) of 3-amino-5-nitro-2,1-benzisothiazole, dissolve it in the above solution, and then add 2ml of concentrated sulfuric acid to obtain a dark brown solution in which all solids are dissolved.
[0069] Take 5ml of concentrated sulfuric acid and 7.5ml of glacial acetic acid, mix it into a 40% sulfuric acid / acetic acid solution in a 250ml three-necked bottle, add a little ice cubes, and put it in an ice-salt bath. Add the above-prepared dark brown solution dropwise into the sulfuric acid solution, and stir rapidly. After the dropwise addition, react for 10-15 minutes.
[0070] Weigh 3mmol 1-(2-chlorophenyl)-3-methyl-5-pyrazolone, put it into a 250ml three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com