Electrolyte additive for improving battery high-temperature gas expansion, electrolyte and lithium ion battery containing electrolyte
An electrolyte additive and electrolyte technology, applied in the field of lithium ion batteries, can solve problems such as deteriorating battery performance, and achieve the effects of improving stability, improving high-temperature cycle life, and improving high-temperature storage flatulence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
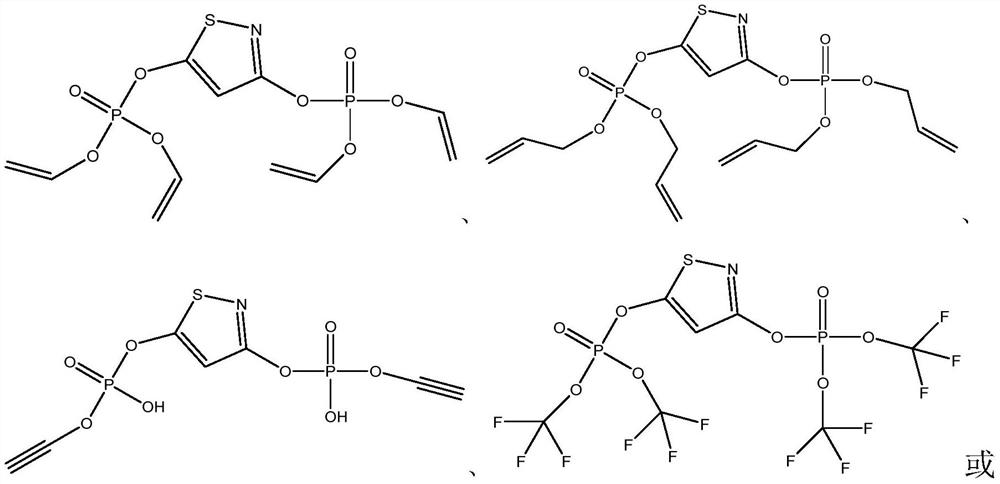
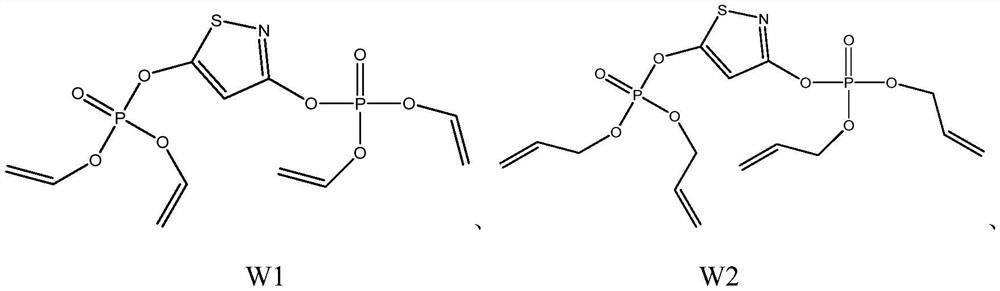
[0034] Preparation of 1,3-diphosphonate-isothiazole compound W1
[0035]Weigh 13.82g (0.1mol) of potassium carbonate and 16.60g (0.1mol) of 1,3-chloroisothiazole into a three-necked flask, add 150mL of freshly distilled acetone and stir to dissolve, then slowly add 7.50g of divinyl phosphate ( 0.05mol) into the flask, reflux reaction for 10-12h, after the reaction is completed, filter to remove solid potassium chloride and potassium carbonate, rotary evaporate, concentrate the solution, add 50mL water and 100mL ether after concentration, shake vigorously for 5min, let stand for half After 1 hour, the ether layer was taken out, and the ether solution was concentrated after repeated extraction twice to obtain 1,3-bisphosphonate-isothiazole compound, which was named W1, and the weight of W1 was 3.64 g (yield 37%).
Embodiment 2
[0037] Preparation of 1,3-diphosphonate-isothiazole compound W2
[0038] Weigh 13.82g (0.1mol) of potassium carbonate and 16.60g (0.1mol) of 1,3-chloroisothiazole into a three-necked flask, add 150mL of freshly distilled acetone and stir to dissolve, then slowly add bis(1,2-propenyl ) Phosphate ester 8.90g (0.05mol) in the flask, reflux reaction 10-12h, after the reaction is completed, filter to remove solid potassium chloride and potassium carbonate, rotary evaporation, concentrated solution, add 50mL water and 100mL ether after concentrating, force Shake for 5 minutes, take out the ether layer after standing for half an hour, repeat the extraction twice and then concentrate the ether solution to obtain 1,3-bisphosphonate-isothiazole compounds, which are named W2, and the weight of W2 is 4.72g (yield 42%).
Embodiment 3
[0040] Preparation of 1,3-diphosphonate-isothiazole compound W3
[0041] Weigh 13.82g (0.1mol) of potassium carbonate and 16.60g (0.1mol) of 1,3-chloroisothiazole into a three-necked flask, add 150mL of freshly distilled acetone and stir to dissolve, then slowly add 7.25g of diethynyl phosphate ( 0.05mol) into the flask, reflux reaction for 10-12h, after the reaction is completed, filter to remove solid potassium chloride and potassium carbonate, rotary evaporate, concentrate the solution, add 50mL water and 100mL ether after concentration, shake vigorously for 5min, let stand for half After 1 hour, the ether layer was taken out, and the ether solution was concentrated after repeated extraction twice to obtain 1,3-bisphosphonate-isothiazole compound, which was named W3, and the weight of W3 was 4.41 g (yield 46%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com