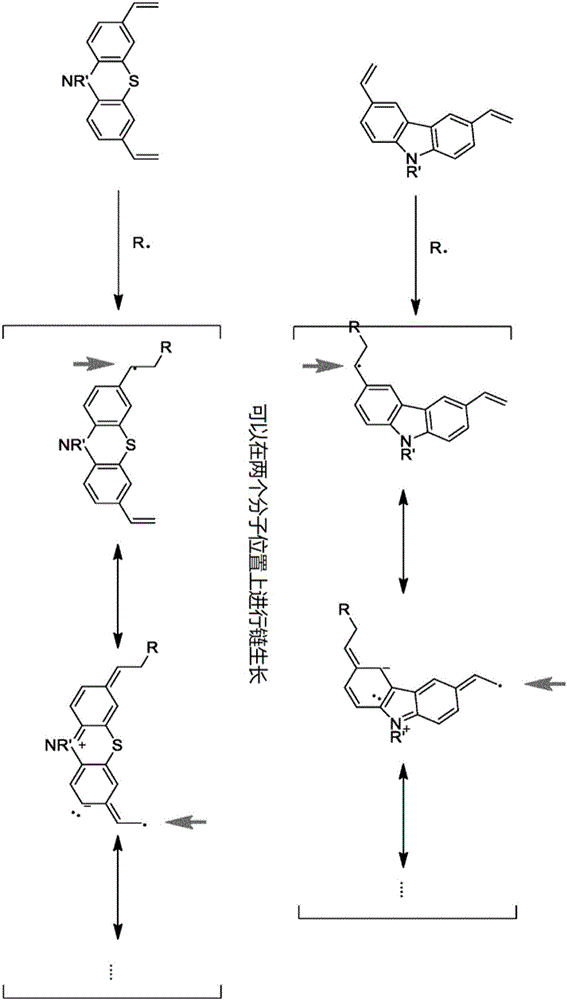
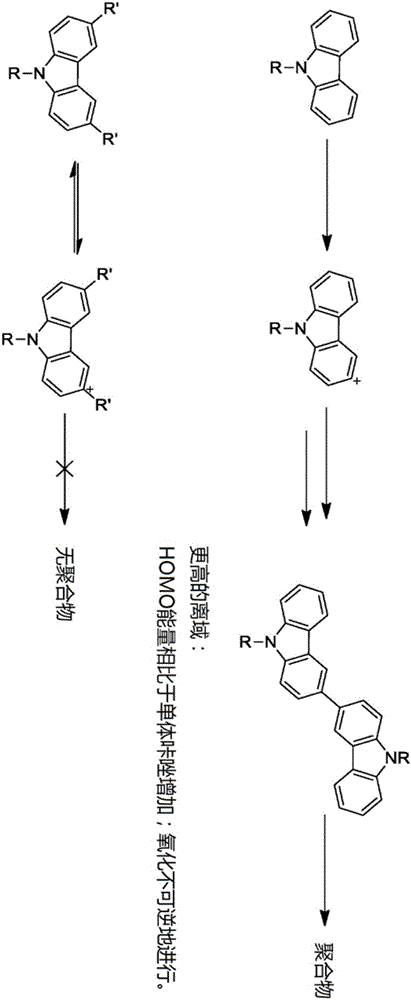
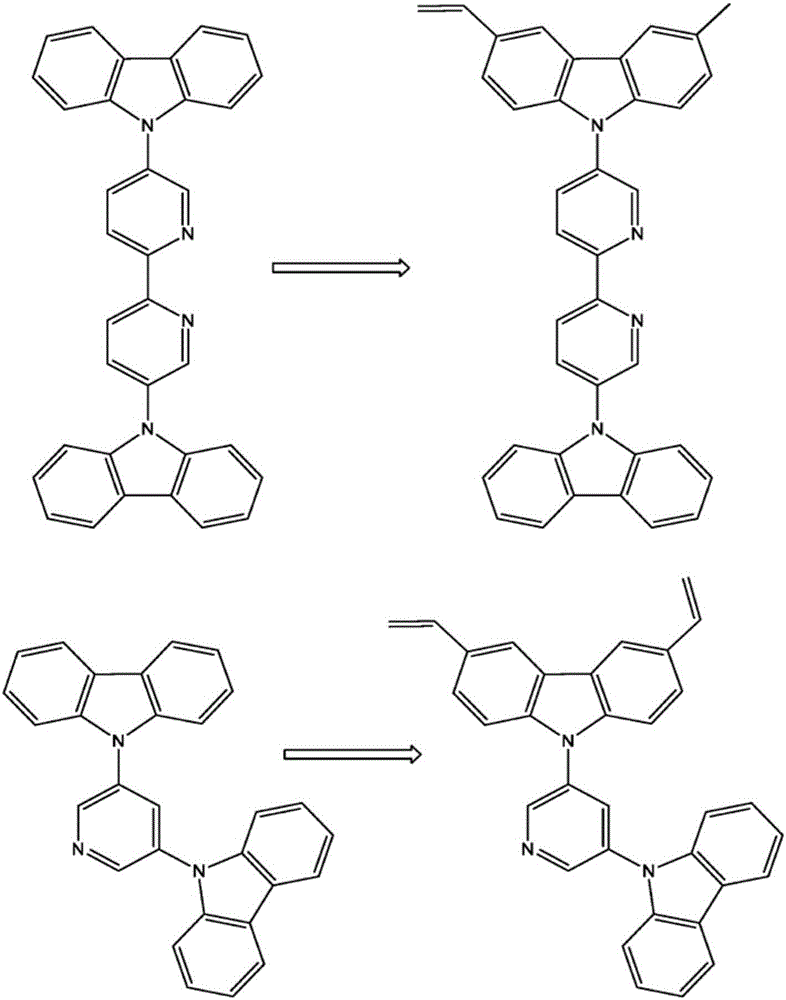
Crosslinkable host materials
A system and donor technology, applied in luminescent materials, electro-solid devices, semiconductor devices, etc., can solve the problems of short operating life, insoluble compounds, and difficult-to-purify materials processing and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Molecules according to the invention
[0110] All molecules with the crosslinkable group ethylene are shown in the list below. The person skilled in the art knows how to introduce alternative crosslinkable groups PG into molecules to obtain other molecules according to the invention.
[0111]
[0112]
[0113]
[0114]
[0115]
[0116]
[0117]
[0118]
[0119]
[0120]
[0121]
Embodiment 2
[0122] Example 2: Synthetic sequence for the synthesis of molecules according to the invention such as 2-N-(carbazolyl)6-N-(3,6-divinylcarbazolyl)-pyridine (molecule 1)
[0123]
[0124] First replacement step:
[0125]Carbazole (10 mmol, 1.00 equiv) was mixed with sodium hydride (60% with paraffin, 500 mg, 13 mmol, 1.3 equiv) and dissolved in portions with a total of 110 mL of dioxane under nitrogen. The rate of addition was adapted to the formation of hydrogen to avoid foaming. After complete addition the reaction was stirred at room temperature for 15 minutes, then heated at reflux for 15 minutes. A clear yellow solution formed which fluoresced strongly under UV light (excitation at 366 nm). 2,6-Difluoropyridine (1.15 g, 10 mmol, 1.00 equiv) was added dropwise to the reaction mixture. Fluorescence was significantly reduced under ultraviolet light. The reaction mixture was heated at reflux until the reactants were completely reacted (reaction controlled by HPLC, GC-...
Embodiment 3
[0136] Example 3: Molecule 2
[0137] 2-N-(carbazolyl)-6-N-(3,6-di-(4-vinylphenyl)-carbazolyl)-pyridine (molecule 2).
[0138]
[0139] The synthesis was carried out similarly to the synthesis of molecule 1, but using (HO) 2 BPhCH=CH 2 as a boron derivative. Beige solid (50%). – 1 H NMR (500MHz, chloroform-d) δ=8.47–8.41(m,2H), 8.25–8.04(m,7H), 7.80–7.62(m,8H), 7.61–7.52(m,4H), 7.47(ddd ,J=8.5,7.2,1.3Hz,2H),7.38(td,J=7.5,1.0Hz,2H),6.82(dd,J=17.6,10.9Hz,2H),5.84(dd,J=17.5,0.9 Hz, 2H), 5.31 (dd, J = 10.9, 0.9Hz, 2H) ppm. – 13 C NMR (126MHz, chloroform-d) δ = 151.6, 151.41, 140.96, 139.49, 139.37, 136.51, 136.17, 134.37, 127.33, 127.0, 126.7, 126.7, 126.4, 125.8, 125.3, 124.6, 128.4, 120.4, 126.4, , 113.7, 112.5, 111.9ppm. –IR(ATR) 2922(vw), 1730(vw), 1625(vw), 1570(m), 1477(m), 1439(vs), 1375(m), 1324(m), 1220(m), 1186(m), 1029(w), 987(vw), 902(w), 841(w), 794(m), 747(s), 721(s), 668(vw), 640(vw), 597(vw),560(vw),499(vw),420(w)cm -1 . –.HRMS(FAB-MS,3-NBA,C 45 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com