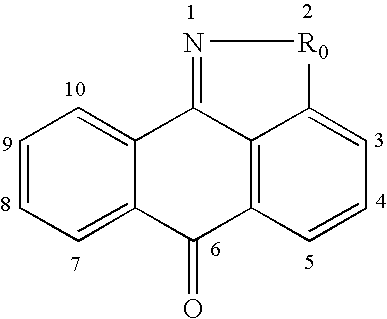
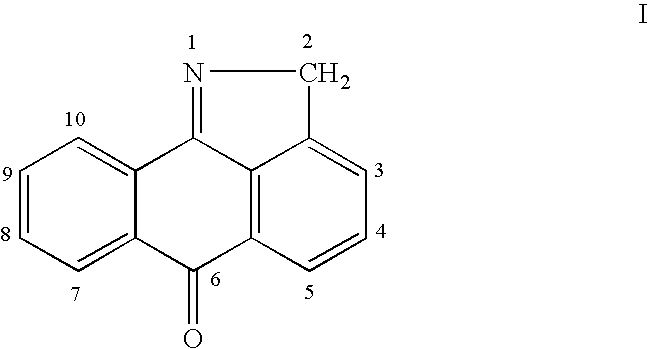
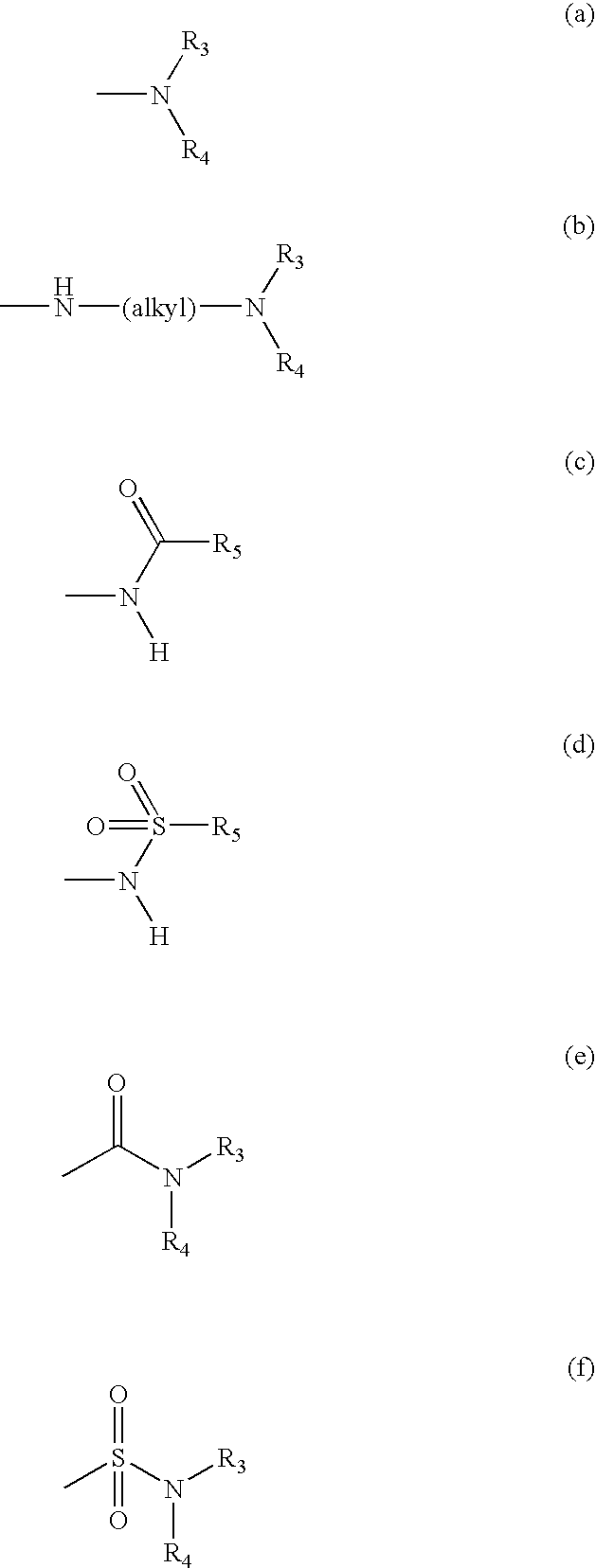
Methods for treating inflammatory conditions or inhibiting JNK
a technology of inflammatory conditions and inhibitors, applied in the direction of antinoxious agents, drug compositions, extracellular fluid disorders, etc., can solve the problems of cell death and scar formation, congestive heart failure, renal failure, or cerebral dysfunction, and selective defect in the ability of th1 effector cells to express ifng
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
5.1 DEFINTIONS
[0094] As used herein, the terms used above have the following meaning:
[0095] An "effective amount" when used in connection with a Compound of Formula I-VI, or a pharmaceutically acceptable salt thereof, is an amount effective for treating or preventing an inflammatory condition, a liver disease, a cardiovascular disease, a neurodegenerative disease or cancer.
[0096] A "patient" includes an animal (e.g., cow, horse, sheep, pig, chicken, turkey, quail, cat, dog, mouse, rat, rabbit or guinea pig), in one embodiment a mammal such as a non-primate and a primate (e.g., monkey and human), and in another embodiment a human. In certain embodiments, the patient is an infant, child, adolescent or adult.
[0097] "Alkyl" means a straight chain or branched, saturated or unsaturated chain having from 1 to 8 carbon atoms. Representative saturated alkyl groups include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, 2-methyl-1-propyl, 2-methyl-2-propyl, 2-methyl-1-butyl, 3-me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com