Benzoisothiazole compound, preparation and uses and disperse dyes composition
A technology of benzisothiazole and dye composition, which is applied in the direction of azo dyes, organic dyes, monoazo dyes, etc., and can solve the problem of not bright enough color and luster
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
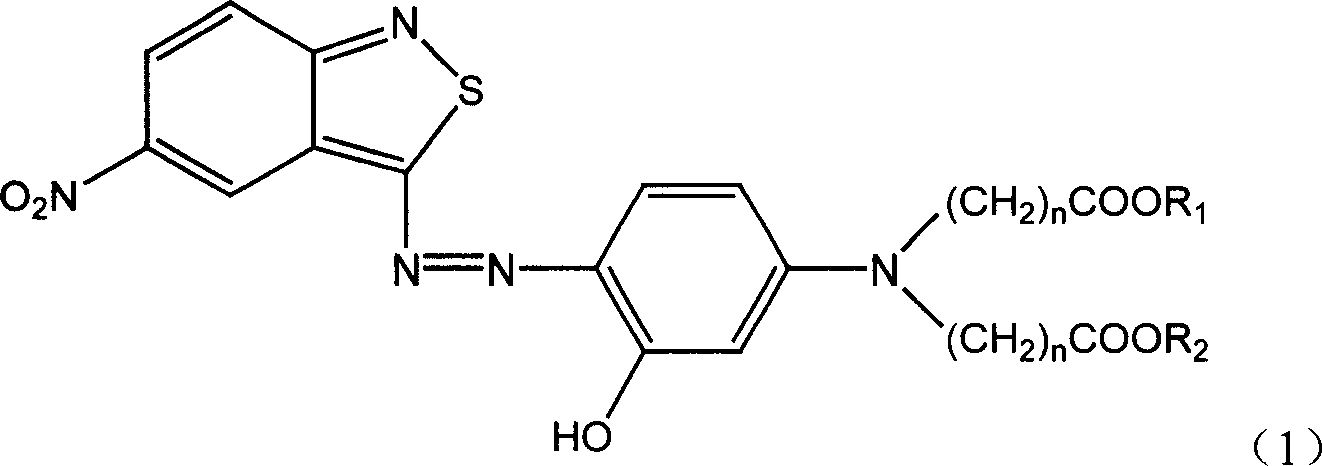
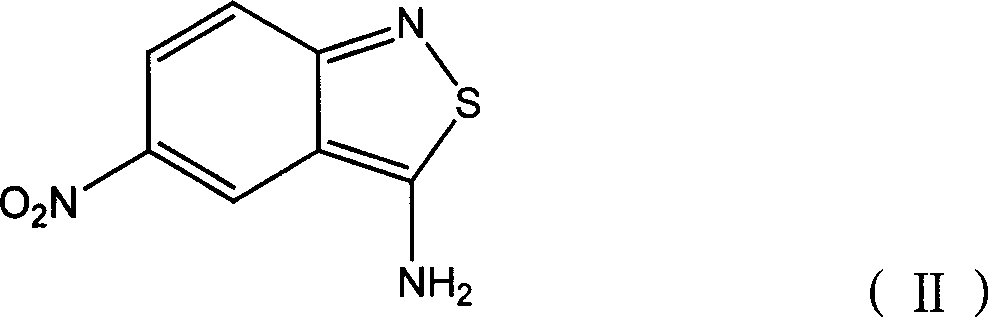
[0028] Add 80g of sulfuric acid (98%) and 80g of 20% nitrosylsulfuric acid into the three-necked flask, stir for 30-60min, control the temperature in an ice-water bath at 0-5°C, and dissolve 0.2mol (39g) of 3 within 2 hours under stirring. -Amino-5-nitro-2,1-benzisothiazole was added to the above-mentioned three-necked flask, and the reaction was kept for about 5 hours, and the diazotization was completed.
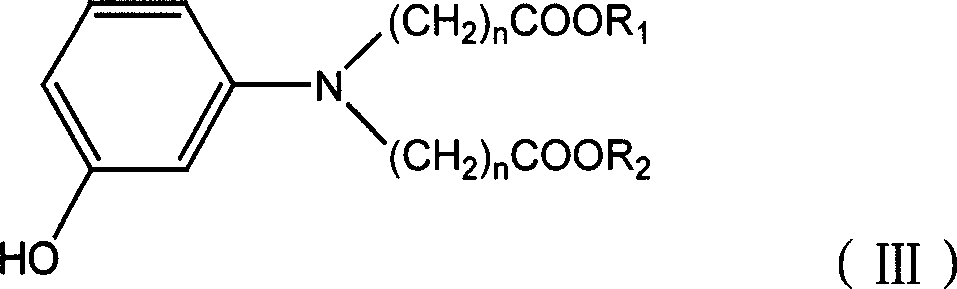
[0029] Add 21.8g of m-aminophenol, 86g of methyl acrylate and 25g of acetic acid into the three-necked flask, heat to 90-95°C, and react for 19 hours. At -35°C, a solution of 3-hydroxy-N,N-dimethoxycarbonylethylaniline was obtained.
[0030] Take the above-mentioned 3-hydroxy-N, N-dimethoxycarbonyl ethylaniline solution, add 300 g of water, 30 g of sulfuric acid (98%), and 2 g of sulfamic acid and an emulsifier, and stir in an ice-water bath with a controlled temperature of 0 to 10 ° C After beating, slowly add the above prepared diazonium solution dropwise later to carry...
Embodiment 2
[0033] According to the preparation method described in Example 1, the difference is that 3-hydroxyl-N, N-dimethoxycarbonylethylaniline is used in an equimolar amount of 3-hydroxyl-N, N-diethoxycarbonylethyl By replacing aniline, the blue dye monomer compound of the following formula (1-b) can be obtained.
[0034] Wherein, 3-hydroxyl-N, N-diethoxycarbonylethylaniline is obtained through the following steps: add 21.8g m-aminophenol, 100g ethyl acrylate and 25g acetic acid, 5g sodium bromide in a three-necked flask, heat to 90 -95°C, after reacting for 19 hours, at this temperature, distill off excess ethyl acrylate and acetic acid under reduced pressure, and lower the temperature to 30-35°C to obtain 3-hydroxy-N,N-diethoxycarbonylethylaniline solution .
[0035]
Embodiment 3
[0037] According to the preparation method described in Example 1, the difference is that 3-hydroxyl-N, N-dimethoxycarbonylethylaniline is used in an equimolar amount of 3-hydroxyl-N, N-dimethoxycarbonylmethyl Substituting aniline, the blue dye monomer compound of the following formula (1-C) can be obtained.
[0038] Wherein, 3-hydroxyl-N, N-dimethoxycarbonylmethylaniline can be obtained through the following steps: add 21.8g m-aminophenol, 75g methyl chloroacetate and 28g sodium carbonate, 5g sodium bromide in there-necked flask, Heat to 115-135°C, react for 5 hours, at this temperature, distill off excess methyl chloroacetate under reduced pressure, cool down to 30-35°C, add 150g of acetic acid to get 3-hydroxy-N,N-dimethoxy Carbonylmethylaniline solution.
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com