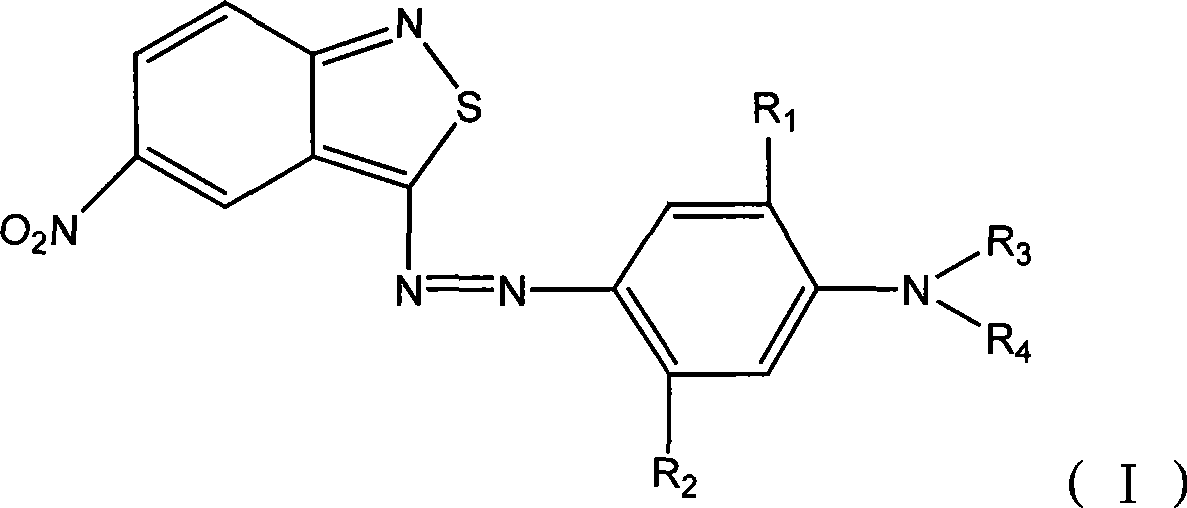
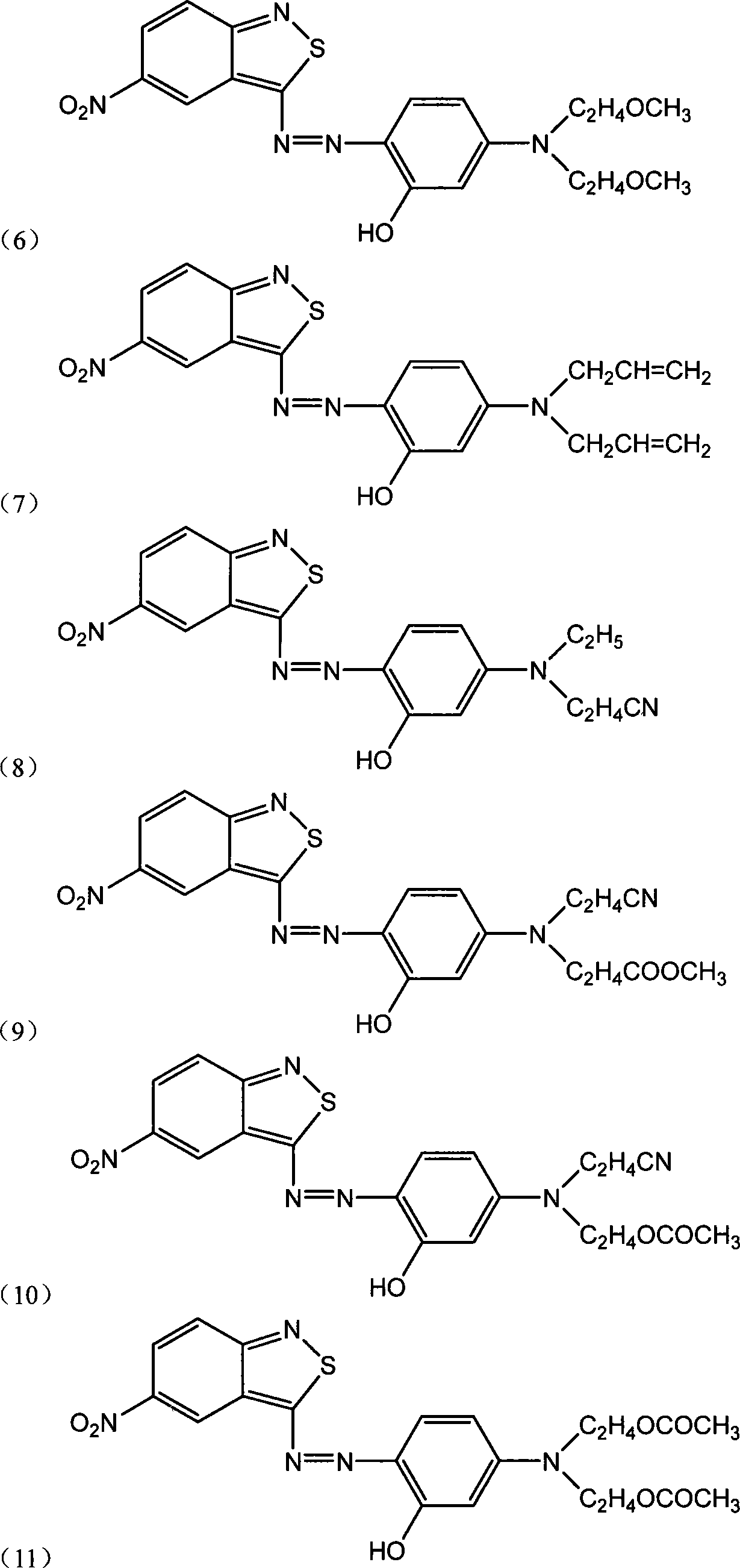
Benzo isothiazole compound, preparation and application and disperse dyes composition
A technology of benzisothiazole and compounds, which is applied in the field of dye monomer compounds and can solve problems such as insufficient color and luster
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 80g of sulfuric acid (98%) and 80g of 20% nitrosylsulfuric acid into the three-necked flask, stir for 30-60min, control the temperature in an ice-water bath at 0-5°C, and dissolve 0.2mol (39g) of 3 within 2 hours under stirring. -Amino-5-nitro-2,1-benzisothiazole was added to the above-mentioned three-necked flask, and the reaction was kept for about 5 hours, and the diazotization was completed.
[0037] Add 21.8g of m-aminophenol, 86g of methyl acrylate and 25g of acetic acid into the three-necked flask, heat to 90-95°C, and react for 19 hours. At -35°C, a solution of 3-hydroxy-N,N-dimethoxycarbonylethylaniline was obtained.
[0038]Take the above-mentioned 3-hydroxy-N, N-dimethoxycarbonyl ethylaniline solution, add 300 grams of water, 30 g of sulfuric acid (98%), and 2 g of sulfamic acid and 5 g of Pingping plus OS-15, and control the temperature in an ice-water bath Stir and beat at 0-10°C, then slowly add the prepared diazonium solution dropwise for coupling re...
Embodiment 2
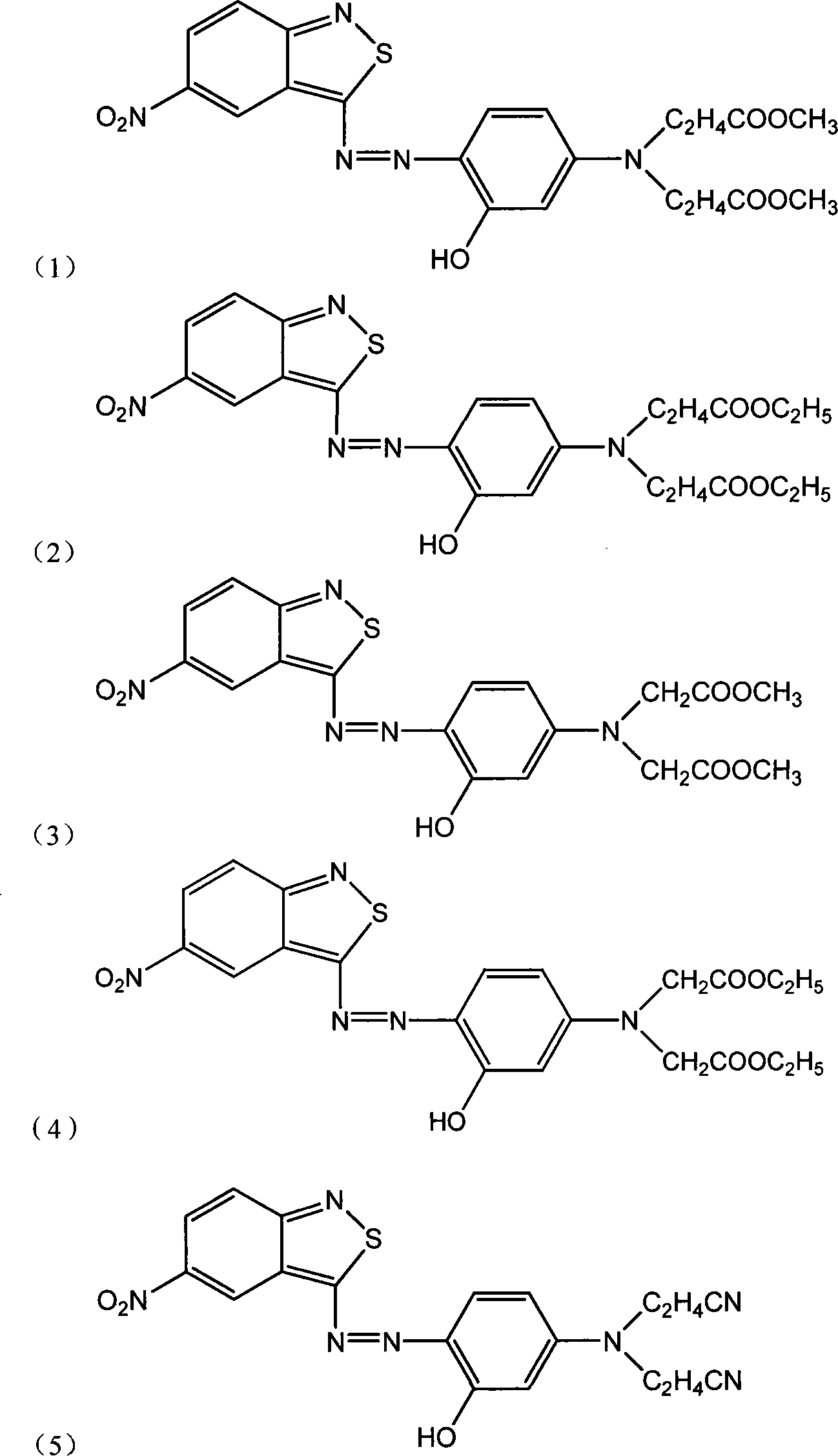
[0043] According to the preparation method and dyeing method of Example 1, the difference is that 3-hydroxyl-N, N-dimethoxycarbonylethylaniline is mixed with an equimolar amount of 3-hydroxyl-N, N-diethoxycarbonylethylaniline Substituted by aniline, the dye monomer compound represented by the following formula (I-2) (which contains a small amount of by-product (I-2')) can be obtained, and blue dyed fabrics with excellent color fastness can be obtained after dyeing treatment.
[0044] Wherein, 3-hydroxyl-N, N-diethoxycarbonylethylaniline is obtained through the following steps: add 21.8g m-aminophenol, 100g ethyl acrylate and 25g acetic acid, 5g sodium bromide in a three-necked flask, heat to 90 -95°C, after reacting for 19 hours, at this temperature, distill off excess ethyl acrylate and acetic acid under reduced pressure, and lower the temperature to 30-35°C to obtain 3-hydroxy-N,N-diethoxycarbonylethylaniline solution .
[0045]
Embodiment 3
[0047] According to the preparation method and dyeing method of Example 1, the difference is that 3-hydroxyl-N, N-dimethoxycarbonylethylaniline is mixed with an equimolar amount of 3-hydroxyl-N, N-dimethoxycarbonylmethyl Substituted by aniline, the dye monomer compound of the following formula (I-3) (which contains a small amount of by-product (I-3')) can be obtained, and a blue dyed fabric with excellent color fastness can be obtained after dyeing treatment.
[0048] Wherein, 3-hydroxyl-N, N-dimethoxycarbonylmethylaniline can be obtained through the following steps: add 21.8g m-aminophenol, 75g methyl chloroacetate and 28g sodium carbonate, 5g sodium bromide in there-necked flask, Heat to 115-135°C, react for 5 hours, at this temperature, distill off excess methyl chloroacetate under reduced pressure, cool down to 30-35°C, add 150g of acetic acid to get 3-hydroxy-N,N-dimethoxy Carbonylmethylaniline solution.
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com