Compound for preventing and treating pain and application thereof
A technology of compound and composition, applied in the field of pain treatment and prevention, can solve problems such as unsatisfactory treatment effect, and achieve good analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
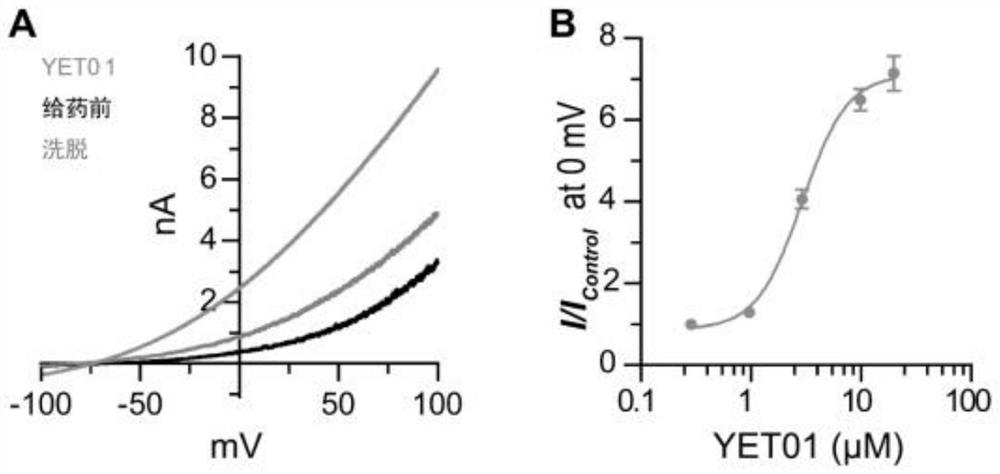
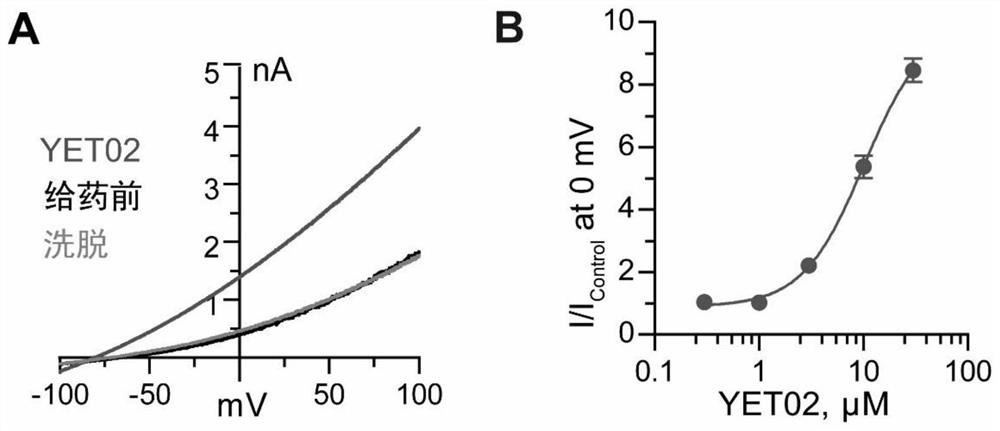
[0087] Whole-cell electrophysiological assays
[0088] The cDNA of human TREK-1 was subcloned into pEGFP-N1 expression vector. The constructed plasmid was transfected into CHO-K1 cells with Lipofectamine 3000. The cell culture medium used in the experiments was DMEM / F12 containing 10% FBS and 1% penicillin-streptomycin. Culture conditions were constant temperature at 37°C, 5% CO 2 gas environment.
[0089] Cells cultured 16-36 hours after transfection can be used for electrophysiological experiments. The detection adopts Whole-CellRecording whole-cell patch clamp recording mode. The experiments used a HEKA EPC10 amplifier, a Sutter Instrument MP-225 micromanipulator, a Nikon fluorescence microscope, and a Bio-Logic RSC-200 drug delivery system. The glass electrode is pulled by SutterInstrument P-97 electrode puller, and the resistance of the liquid is 3-7MΩ. TREK-1 intracellular fluid components include: 140mM KCl, 2mM MgCl 2 , 10mM EGTA, 1mM CaCl 2 , 10mM HEPES, and a...
Embodiment 2
[0093] Animal pharmacology and drug efficacy experiment
[0094] The test drug used in this embodiment includes:
[0095] YET01 was synthesized by conventional methods, and pregabalin was commercially available.
[0096] In the embodiment of YET01, two doses are set, which are 20mg / kg and 50mg / kg respectively. The way of administration is oral gavage, 10ml / kg.
[0097] Compound Dissolution:
[0098] YET01 was dissolved according to the following steps: (1) 20% injection volume of DMSO was completely dissolved; (2) 20% injection volume polyethylene glycol 400 was added and mixed evenly; (3) 60% injection volume was diluted with sterile normal saline to the final volume. According to the sequence of operations, mediate for 30 seconds in each step, and then ultrasound for 1 minute, and configure it for use on the same day.
[0099] Solvent control group: DMSO: polyethylene glycol 400: normal saline = 1:1:2
[0100] Positive control drug:
[0101] Pregabalin: The solvent is...
Embodiment 21
[0106] Embodiment 2.1 mouse thermal stimulation tail flick experiment (tail immersion)
[0107] In the mouse tail flick experiment, the mouse tail was immersed in 52°C water, and the mouse raised its tail because it felt the pain caused by the noxious heat stimulus, and the pain tolerance of the mouse was judged by timing the tail-raising time latency. Such as Figure 4 As shown, the results of this experiment show that YET01 can effectively relieve the pain caused by noxious heat stimulation in mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com