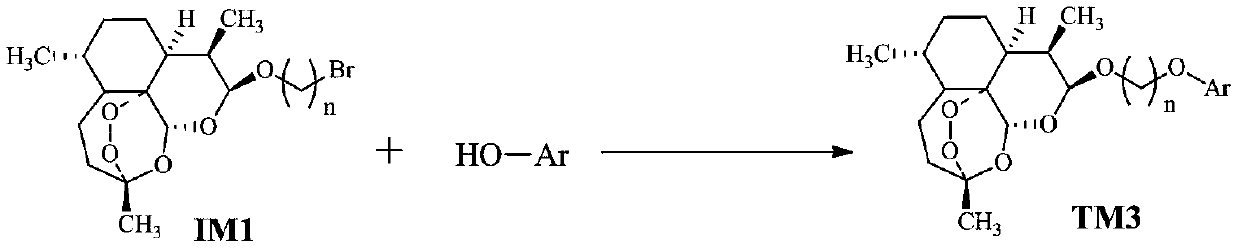Simple phenol conjugates of dihydroartemisinin, synthetic method and application
A technology of dihydroartemisinin and synthesis method, applied in the field of chemical medicine, achieving good application prospects, simple synthesis method, and good Wnt signaling channel activation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1, Preparation of simple phenolic conjugates of dihydroartemisinin
[0027] (1) The preparation method of intermediate IM1 is as follows:
[0028]
[0029] Add dihydroartemisinin (DHA), diethyl ether and bromohydrin, and add boron trifluoride-diethyl ether (BF 3 ·Et 2 O), under stirring reaction 5 ~ 20h, after the completion of the reaction, add saturated NaHCO 3 The reaction was terminated, the layers were left to stand, the aqueous layer was extracted with ethyl acetate (EtOAc, or EA), the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 Dry, filter with suction, and remove the solvent from the filtrate under reduced pressure to obtain a crude product, which is recrystallized with petroleum ether (PE)-EA mixed solvent to obtain intermediate IM1.
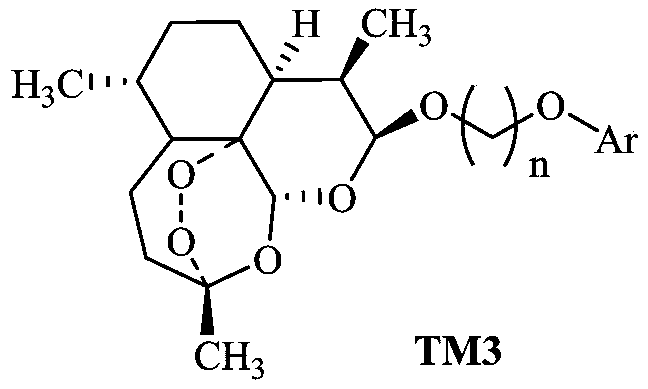
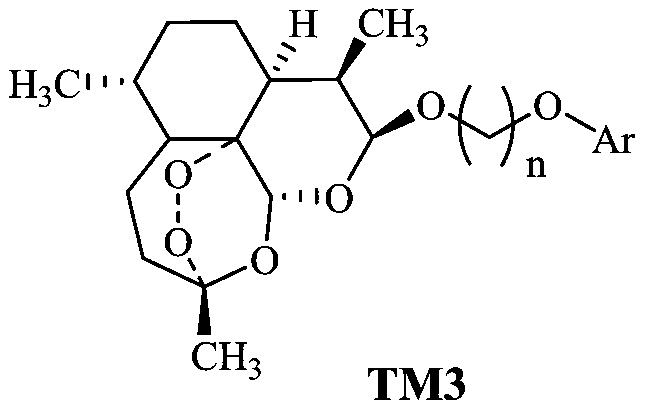
[0030] (2) DHA simple phenolic conjugates, we use TM3 to represent in this application, its preparation method is as follows:
[0031] In the 100mL reaction bottle, add the raw mater...
Embodiment 2
[0033] Example 2, the preparation results of dihydroartemisinin simple phenolic conjugates
[0034] When n=2 or 3 according to the preparation method described in Example 1, Ar is
[0035]
[0036] When one of them was used, a series of products TM3-1 to TM3-21 were prepared, and the respective reaction conditions, yields, product yields, and product melting points are shown in Table 1.
[0037] Table 1 Experimental results of synthesis of TM3 series compounds
[0038]
[0039]
[0040] Example 3, Characterization data of simple phenolic conjugates of dihydroartemisinin
[0041] A series of products of TM3-1~TM3-21 obtained in embodiment 2 were carried out 1 H NMR (AV-300), 13 C NMR (AV-300) and HR MS (Varian 7.0T) test and characterization, the molecular structure and test data are as follows:
[0042]
[0043] TM3-1(3R,6R,8aS,9R,10S,12R,12aR)-3,6,9-Trimethyl-10-(3-phenoxypropoxy)decahydro-12H-3,12-epoxy[1,2]dioxepino[ 4,3-i]isochromene
[0044] 1 H NMR (...
Embodiment 4
[0090] Embodiment 4, TM3 target molecule anti-tuberculosis activity test
[0091] U.S. Eli Lilly and Company (Eli Lilly and Company) company tested the anti-tuberculosis activity of the TM3-1~TM3-21 sample that embodiment 2 prepared, at first test the percentage inhibitory rate of single-concentration sample to Mycobacterium tuberculosis; Secondly, highly active molecules are screened out for multi-concentration testing; finally, a variety of cells are tested. The test results are shown in Table 2.
[0092] Table 2 TM3 series target molecules against Mycobacterium tuberculosis H 37 Inhibition rate of Rv
[0093]
[0094]
[0095] It can be seen from Table 2 that at the test concentration of 20 μM, 2 of the 21 compounds had an anti-tuberculosis activity of more than 40%, showing a moderate inhibitory effect on Mycobacterium tuberculosis; at the same time, it was found that the activity of 3-carbon Linker was higher than that of The activity of the linker is weak. Aryl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com