Method for simultaneously detecting various gene impurities of vilanterol triphenylacetate
A technology for vilanterol triphenylacetate and impurities, which is applied in the field of analytical chemistry, can solve the problems of high sensitivity requirements of detection methods, difficult quantitative detection, and difficult separation, achieves a good linear relationship, ensures product quality, and has high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] Preparation of test solution I: Weigh 305.52 mg of vilanterol triphenylacetate raw material, put it in a 10ml measuring bottle, add 60 μl of VL02imp4, VL02imp6, VL02imp8 positioning solution respectively, add methanol to dilute to the mark, shake well, Instantly. (Preparation of localization solution: Weigh 5.24mg of VL02imp4, accurately add 1ml of methanol to dissolve, and then obtain VL02imp4 localization solution. Prepare VL02imp6 and VL02imp8 localization solutions in the same way, and weigh 5.54mg and 5.78mg respectively.)
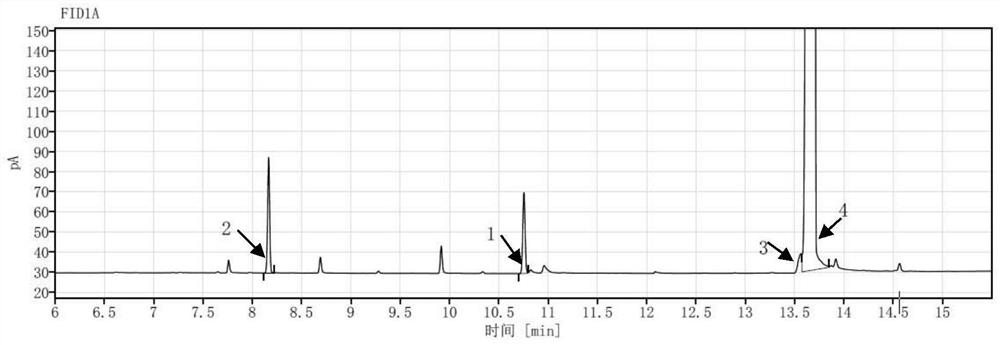
[0100] Measure need testing solution I with described chromatographic condition 1, record chromatogram, see figure 1 . figure 1 It is shown that the VL02imp8 and triphenylacetic acid chromatographic peaks cannot be baseline separated.
[0101] On the basis of chromatographic condition 1, the screening of chromatographic columns was carried out. Replace the capillary chromatographic column (DB-WAX, 0.32mm×30m, 0.5μm) with polyethylene glycol ...
Embodiment 1
[0121] Embodiment 1 Methodological verification of the detection method established by the present invention
[0122] 1.1 Specificity and system adaptability
[0123] Solution preparation:
[0124] (1) Blank solution: Take methanol (chromatographically pure) to get it.
[0125] (2) Test solution: Weigh 30 mg of vilanterol triphenylacetic acid (batch number: VL1709903, source: Livzon Synthetic Pharmaceutical Co., Ltd.), weigh it accurately, put it in a sample injection vial, add 1 ml of methanol to dissolve, and shake well , that is.
[0126] (3) Mixed reference substance stock solution: Take 15 mg each of VL02imp4, VL02imp6 and VL02imp8 reference substances, weigh them accurately, put them in the same 50ml measuring bottle, dissolve them with methanol and dilute to the mark to obtain the product. See Table 1 for the sample weight of each impurity reference substance.
[0127] Table 1 The weighing amount of three impurity reference substances
[0128] Impurity re...
Embodiment 2
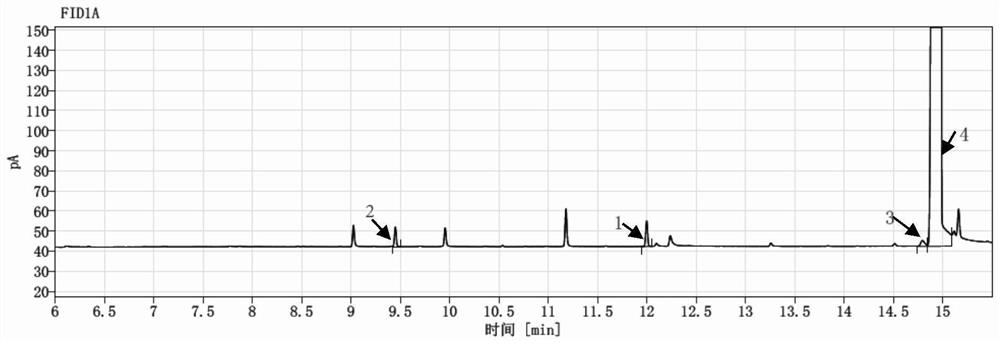
[0180] Example 2 Determination of genotoxic impurities in different batches of vilanterol triphenylacetic acid
[0181] Chromatographic conditions:
[0182] Preparation of the test solution: Weigh 30 mg of vilanterol triphenylacetic acid, accurately weigh it (see Table 12 for the specific weighing amount), put it in a sample injection vial, add 1 ml of methanol to dissolve, shake well, and obtain.
[0183] Table 12 Vilanterol triphenylacetic acid sample volume records
[0184] batch number VL1709901 VL1709902 VL1709903 Sample weight (mg) 29.91 31.41 31.87
[0185] Preparation of reference substance solution: Weigh 14.34mg of VL02imp6 reference substance in a 50ml measuring bottle, dilute to the mark with methanol, and shake well; accurately measure 2ml in a 20ml measuring bottle, dilute to the mark with methanol, shake well, that is have to.
[0186] Determination: Accurately draw 1 μl each of the reference substance solution and the test solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com