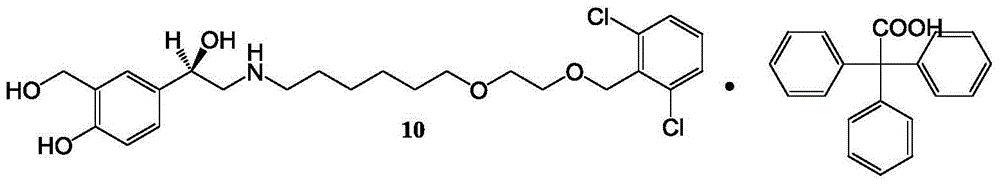
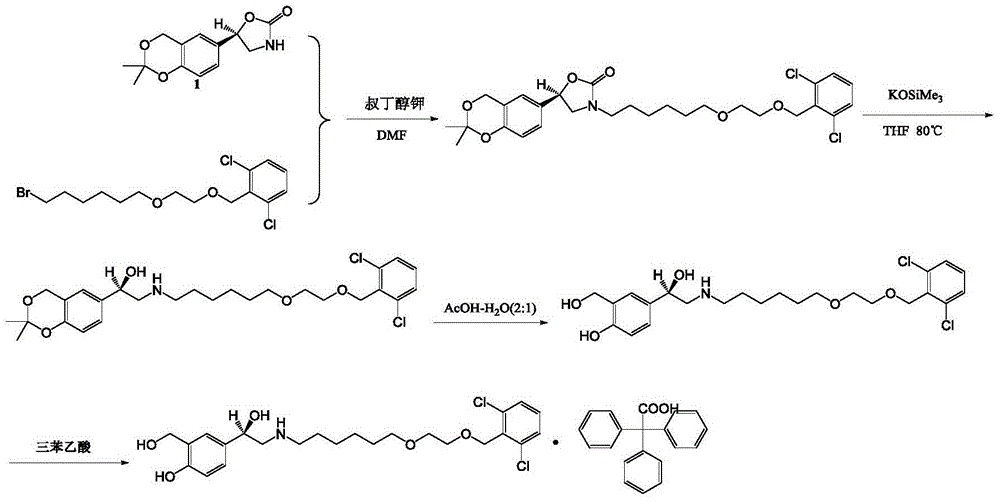
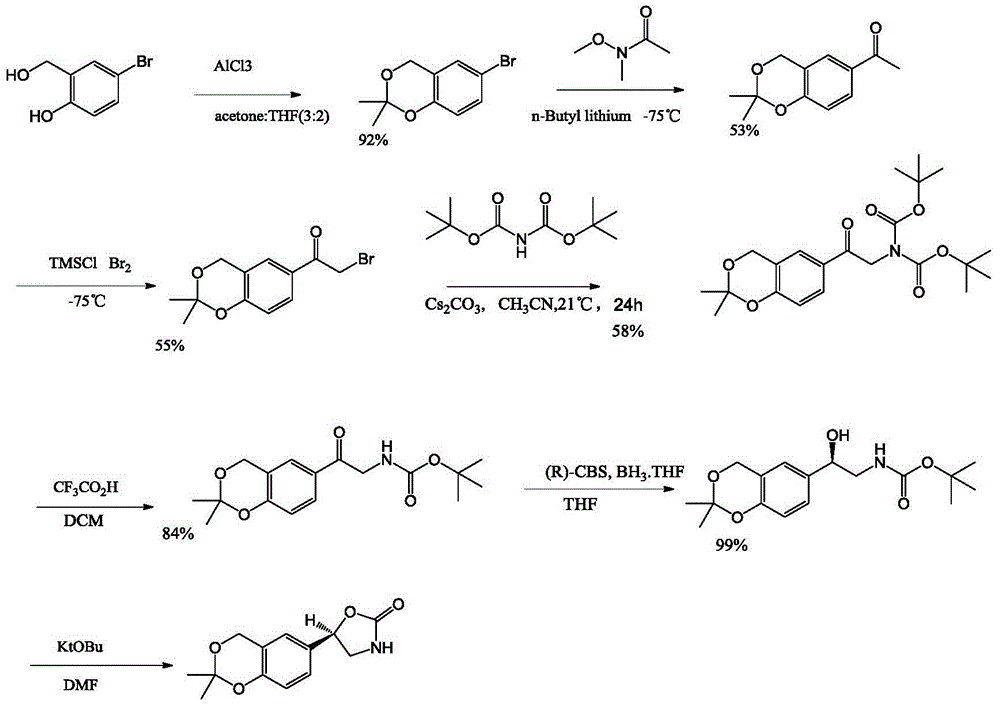
Vilanterol intermediate, preparation method and application thereof
A compound and solvent technology, applied in the field of preparation of vilanterol and its key intermediate -5--1,3 oxazolidin-2-one, can solve the problems of low atom utilization, high price and high cost , to achieve the effect of increasing yield and atom utilization, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of 5-(2-bromoacetyl)-2-hydroxybenzaldehyde 4
[0049] Under nitrogen protection, under an ice bath, disperse 164g (5eq) of aluminum trichloride into 600mL (20 times the amount) of DCM, and slowly add 99.4g (2eq) of bromoacetyl bromide dropwise. After 20min, the temperature rises to room temperature, and the reaction 1h, 30g of salicylaldehyde was added dropwise to the mixture, and the dropwise addition was completed in 20min, and the reaction was carried out overnight at 35°C. Ice water was added to the reaction solution, and the organic layer was separated, washed with water, dried, and then concentrated to dryness in vacuo. Recrystallize with DCM and n-hexane, and filter to obtain 36.5 g of the product, with a yield of about 61%. 1 HNMR (400MHz, CDCl 3 ): δ11.52(s, 1H), 9.99(s, 1H), 8.30(s, 1H), 8.17(d, 1H, J=8Hz), 7.10(d, 1H, J=8Hz), 4.39(s , 2H); MS (-ESI) m / z 240 [M-H] -
Embodiment 2
[0050] Example 2: Preparation of 2-bromo-1-[4-hydroxyl-3-(hydroxymethyl)-phenylethan-1-one 5
[0051] 40.0 g of compound 4 was dissolved in 400 mL of acetic acid (10 times the amount), and 6.8 g (1.1 eq) of sodium borohydride was added in batches under ice cooling. After the addition was complete, the reaction was carried out at room temperature for 1 h, and TLC showed that the reaction was complete. Concentrate in vacuo to remove most of the acetic acid, dilute with water, neutralize with sodium bicarbonate, extract with EA, wash the organic phase with water and brine in turn, dry over anhydrous sodium sulfate, and concentrate in vacuo to obtain the crude off-white powder. After reflux washing with DCM, 32 g of white powder was obtained, and the yield was 80%.
[0052] 1 HNMR (400MHz, DMSO-d6): δ10.53(s, 1H), 7.99(s, 1H), 7.79(d, 1H, J=8Hz), 6.87(d, 1H, J=8Hz), 4.75(s , 2H), 4.50(s, 2H); MS(+ESI) m / z 267[M+Na] +
Embodiment 3
[0053] Example 3: Preparation of hydrochloride (6) of 2-amino-1-[4-hydroxyl-3-(hydroxymethyl)-phenylethan-1-one
[0054] 10.0 g of compound 5 was added to 200 mL of ethyl acetate, 6.2 g of urotropine (1.1 eq) was added, and the reaction was carried out at room temperature for 1 h. TLC showed that the reaction was complete. After filtering, the filter cake was vacuum-dried to 15.6 g of white powder. Dissolve the above white powder in 150mL of ethanol, add 17.5mL of concentrated hydrochloric acid (5eq), react at room temperature overnight, and concentrate the reaction solution in vacuo to obtain 16.0g of off-white powder (mixture), which is directly used in the next step.
[0055] 1 HNMR (400MHz, DMSO-d6): δ10.89(s, 1H), 8.40(s, 2H), 7.98(d, 1H, J=2Hz), 7.70(dd, 1H, J=8Hzand2Hz), 7.02(d , 1H, J=8Hz), 4.49(s, 2H), 4.43(s, 2H); MS(+ESI) m / z 182[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com