Vilanterol intermediate as well as preparation method and application thereof
A technology of intermediates and compounds, applied in the field of vilanterol intermediates and its preparation, can solve the problems of difficult control of the quality of intermediates and finished products, inaccurate measurement of oily substances, unfavorable industrial production, etc., to achieve good quality and easy operation , high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0058] Add 40 mL of ethyl acetate, 2.9 g of triphenylacetic acid and 5.5 g of the compound of formula II to the reaction flask, stir the reaction at 70 ° C, after the reaction is completed, drop to 25 ° C for crystallization for 2 h, then crystallize at 5 ° C for 2 h, filter to obtain The white solid was 7.7 g, the yield was 94.5%, and the HPLC purity was 99.2%.
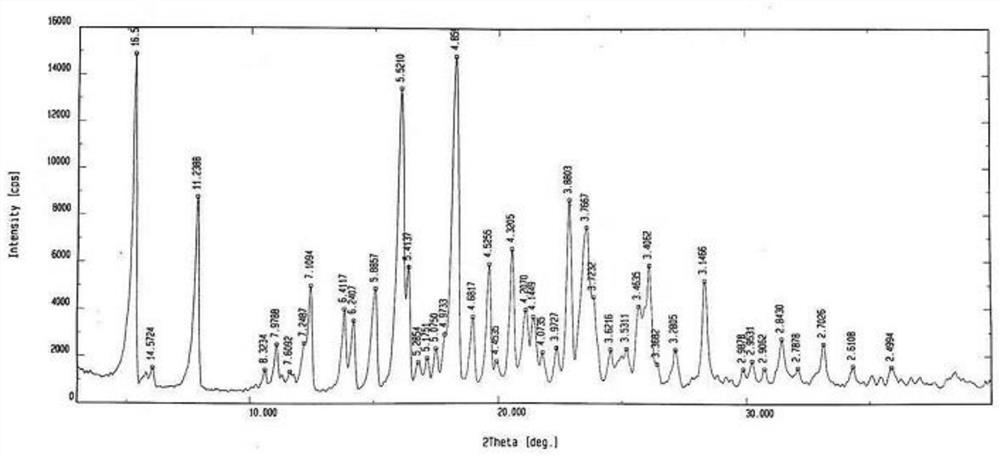
[0059] The white solid obtained in Example 1-1 was subjected to X-ray powder diffraction measurement, and the measured characteristic peak positions were 2θ=5.3°, 7.8°, 12.4°, 13.8°, 14.1°, 15.0°, 16.0°, 16.3°, 18.2 °, 18.9°, 19.6°, 20.5°, 21.1°, 21.4°, 22.9°, 23.6°, 23.8°, 25.7°, 26.1°, 28.3°, such as figure 1 shown.
Embodiment 1-2
[0061] Add 30mL of ethanol, 2.9g of triphenylacetic acid and 5.5g of the compound of formula II to the reaction flask, stir the reaction at 65°C, after the reaction is completed, drop to 30°C for crystallization for 3h, then crystallize at 0°C for 1.5h, filter to obtain white The solid was 7.5 g, the yield was 92.0%, and the HPLC purity was 99.5%.
Embodiment 1-3
[0063] Add 60mL of methanol, 2.9g of triphenylacetic acid and 5.5g of the compound of formula II to the reaction bottle, stir the reaction at 60°C, and then drop to 20°C for 1.5h of crystallization after the reaction, then crystallize at 10°C for 3h, filter to obtain white The solid was 7.5 g, the yield was 92.0, and the HPLC purity was 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com