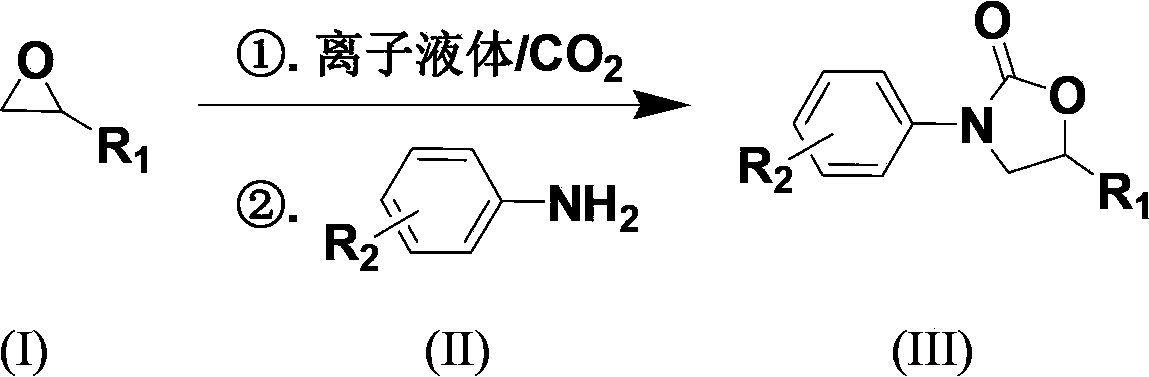
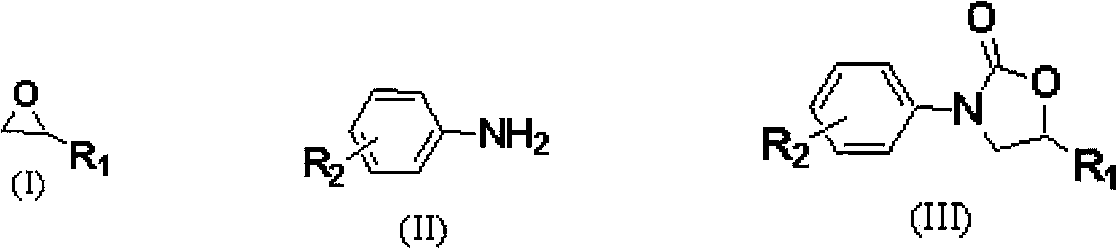Carbon dioxide one-pot method for directly preparing oxazolidine-2-one compounds
A ketone compound, carbon dioxide technology, applied in the direction of organic chemistry, can solve the problem of high raw material price, achieve the effect of low raw material price, enhanced electrophilicity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
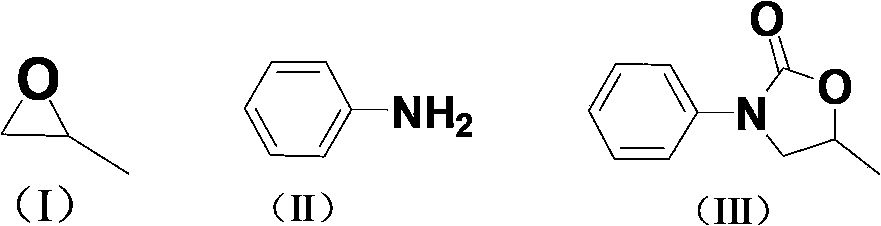
[0025] Embodiment 1: 1-butyl-3-methylimidazole acetate ([Bmim]OAc) catalyzes propylene oxide, CO 2 Preparation of 5-methyl-3-phenyloxazolidin-2-one by one-pot method with aniline
[0026]The first step is to add ionic liquid 1-butyl-3-methylimidazole acetate ([Bmim]OAc) (0.8mmol, 0.159g) and the ring represented by formula (I) into a 50ml autoclave with magnetic stirring. Oxypropane (40mmol, 2.323g), filled with CO at room temperature 2 To the initial pressure of 2.5MPa, heated to 110 ° C for 6 hours. The autoclave was cooled to room temperature and reduced to normal pressure, and the reaction solution was transferred to a 5 mL round bottom flask. The second step adds aniline (2mmol, 0.186g) shown in formula (II), N 2 The reaction was continued at 140° C. for 9 hours under protection. After the reaction, cool to room temperature, use petroleum ether / ethyl acetate = 3:1 as the developer column chromatography, and vacuum dry to obtain 5-methyl-3-phenyloxazolidine- 2-ketone ...
Embodiment 2
[0028] Embodiment 2: 1-butyl-3-methylimidazole acetate ([Bmim]OAc) catalyzes epichlorohydrin, CO 2 One-pot preparation of 5-chloromethyl-3-phenyloxazolidin-2-one with aniline
[0029] In the preparation method of this example, the epoxy compound is epichlorohydrin (40mmol, 3.700g) shown in formula (I), and other preparation conditions and methods are the same as in Example 1, and 5-chlorohydrin shown in formula (III) is prepared. Methyl-3-phenyloxazolidin-2-one 0.423 g, yield 99%.
[0030]
Embodiment 3
[0031] Embodiment 3: 1-butyl-3-methylimidazole acetate ([Bmim]OAc) catalyzes propylene oxide, CO 2 One-pot preparation of 3-(4-chlorophenyl)-5-methyl-oxazolidin-2-one with p-chloroaniline
[0032] In the preparation method of this example, aniline or aniline derivatives are p-chloroaniline (2mmol, 0.255g) shown in formula (II), and other preparation conditions and methods are the same as in Example 1, and 3-chloroaniline shown in formula (III) is prepared. (4-Chlorophenyl)-5-methyl-oxazolidin-2-one 0.389 g, yield 92%.
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com