Method for removing protective groups and preparing dimethoxy taxane compound
A technology for dimethoxytaxane and deprotection group, which is applied in the field of preparation of dimethoxytaxane compounds by deprotection group, can solve the problems of reducing reaction yield and product purity, and achieve improvement of reaction yield and product purity, simple operation, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dissolve p-toluenesulfonic acid (5.0 g, 26.3 mmol) in 500 mL of methanol, add 100.0 g of 200-300 mesh silica gel, stir well at room temperature for 1 hour, and rotary evaporate to dryness to obtain acid-activated silica gel.
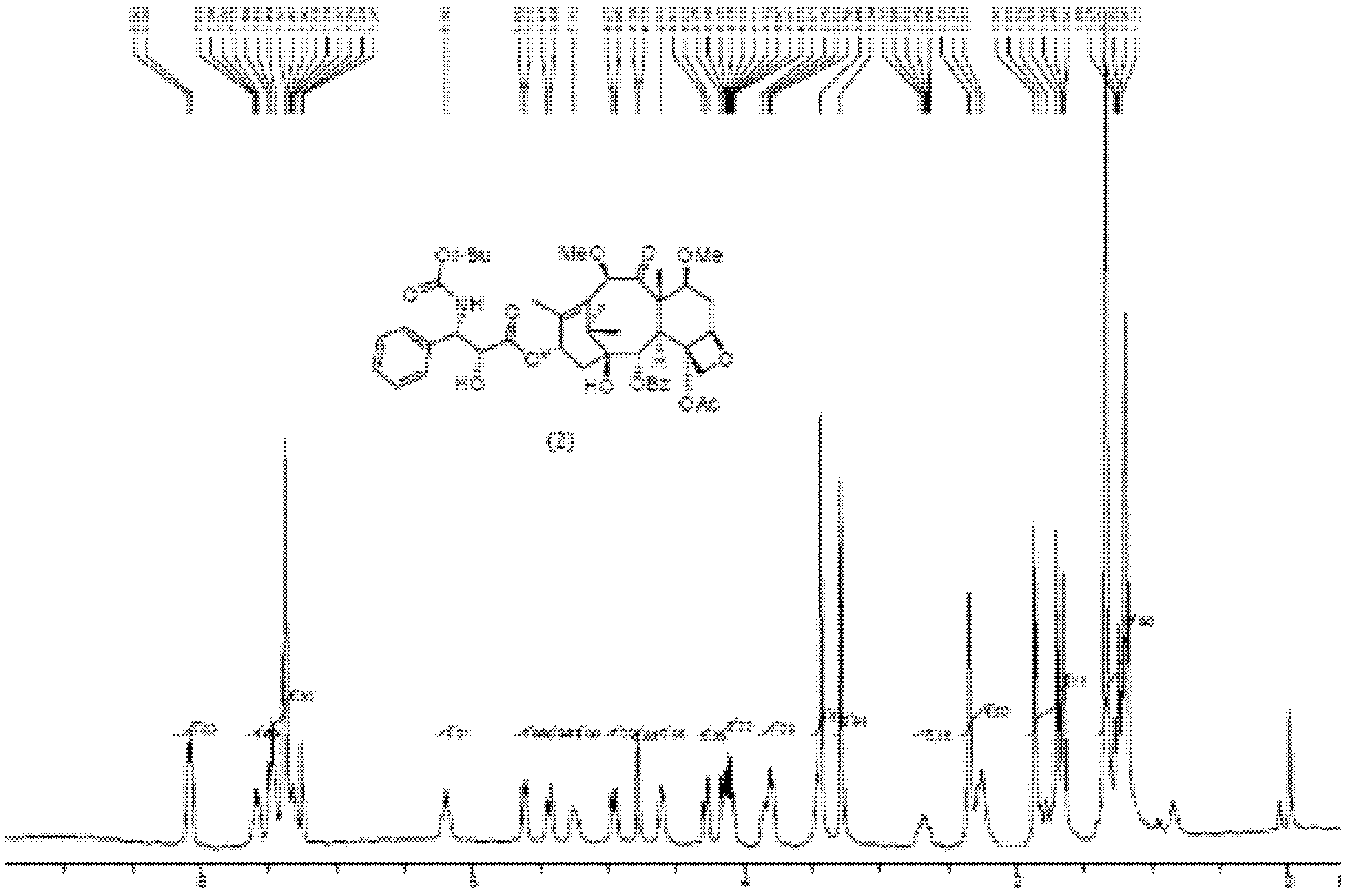
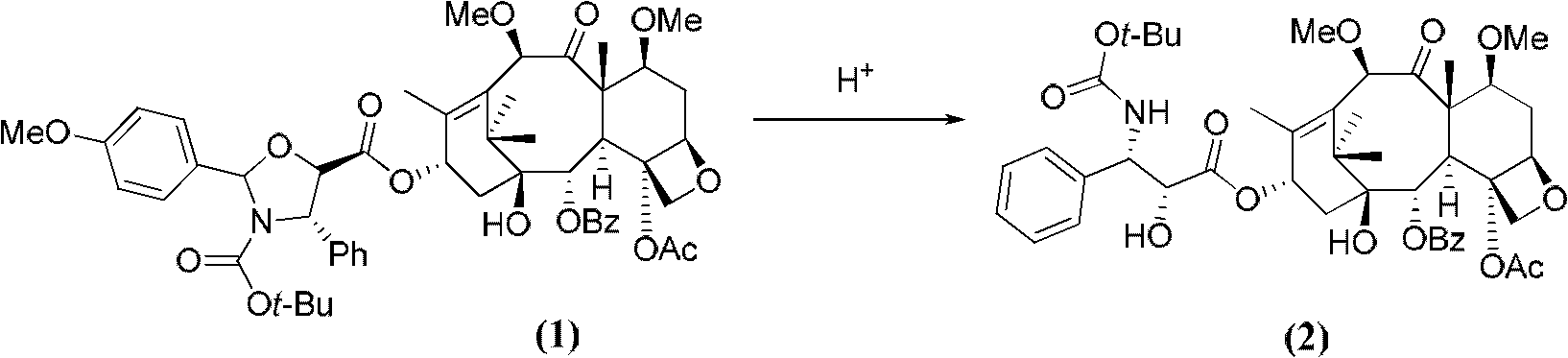
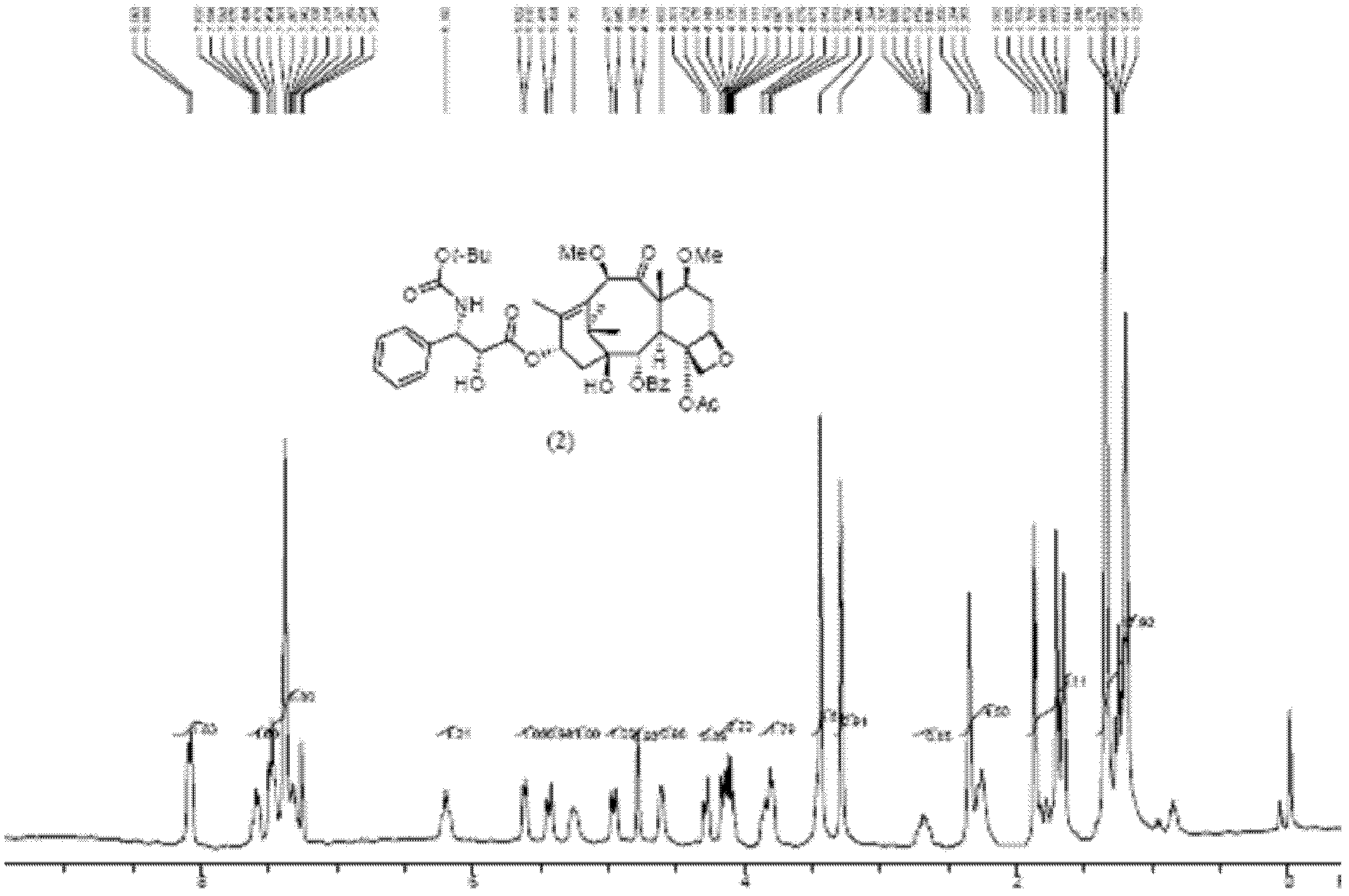
[0019] Dissolve 12.00 g of the prepared acid-activated silica gel in 500 mL of methanol, add compound (1) (10.00 g, 10.5 mmol), and react at room temperature for 1 hour. TLC shows that the reaction is complete. The reaction solution was filtered with suction, and the filtrate was concentrated to dryness to obtain a crude product of dimethoxytaxane compound (2), which was obtained through 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 1: 1) to obtain dimethoxytaxane compound (2). Methoxytaxane compound (2) (8.58g, 10.3mmol), yield 97.8%, purity 95.6%.
Embodiment 2
[0021] Dissolve camphorsulfonic acid (6.1 g, 26.3 mmol) in 500 mL of methanol, add 100.0 g of 200-300 mesh silica gel, stir well at room temperature for 1 hour, and rotary evaporate to dryness to obtain acid-activated silica gel.
[0022] Dissolve 12.00 g of the prepared acid-activated silica gel in 500 mL of methanol, add compound (1) (20.00 g, 21.0 mmol), and react at room temperature for 1 hour. TLC shows that the reaction is complete. The reaction solution was filtered with suction, and the filtrate was concentrated to dryness to obtain a crude product of dimethoxytaxane compound (2), which was obtained through 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 1: 1) to obtain dimethoxytaxane compound (2). Methoxytaxane compound (2) (17.1 g, 20.5 mmol), yield 97.5%, purity 96.5%.
Embodiment 3
[0024] Dissolve concentrated sulfuric acid (2.6 g, 26.3 mmol) in 500 mL of methanol, add 100.0 g of 200-300 mesh silica gel, stir well at room temperature for 1 hour, and rotary evaporate to dryness to obtain acid-activated silica gel.
[0025] Dissolve 12.00 g of the prepared acid-activated silica gel in 500 mL of methanol, add compound (1) (10.00 g, 10.5 mmol), and react at room temperature for 1 hour. TLC shows that the reaction is complete. The reaction solution was filtered with suction, and the filtrate was concentrated to dryness to obtain a crude product of dimethoxytaxane compound (2), which was obtained through 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 1: 1) to obtain dimethoxytaxane compound (2). Methoxytaxane compound (2) (8.58g, 10.3mmol), yield 97.7%, purity 95.8%.
[0026] The resulting product is subjected to nuclear magnetic resonance testing.
[0027] NMR spectrum such as figure 1 Shown: 1 H-NMR (400MHz: CDCl 3 ; chem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com