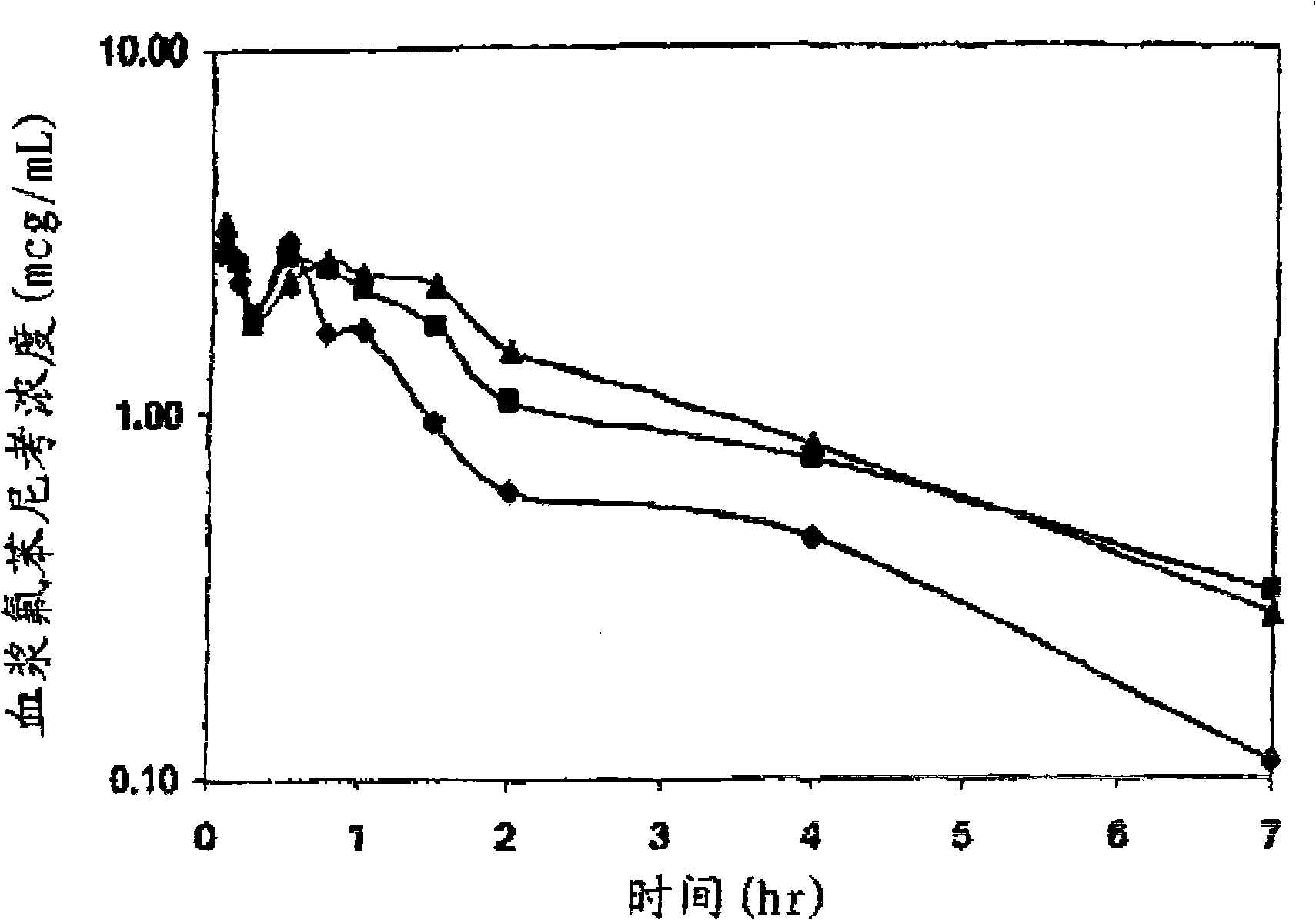
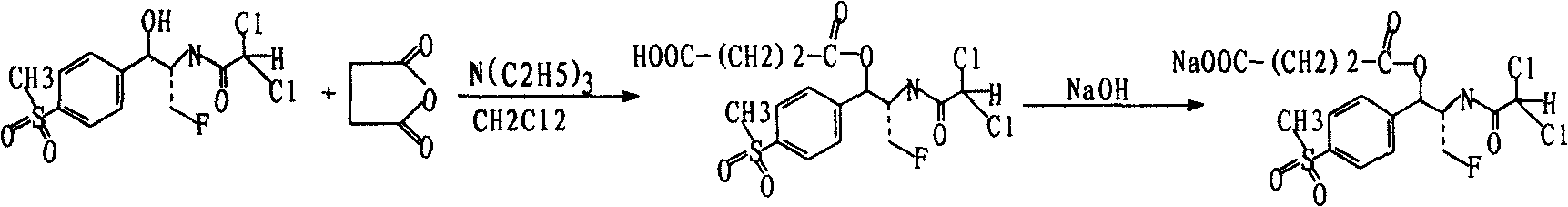
Preparation of florfenicol sodium succinate
A technology of florfenicol succinate and florfenicol, which is applied in chemical instruments and methods, preparation of organic compounds, pharmaceutical formulations and other directions, can solve problems such as difficult industrialized production, harsh chemical synthesis reaction conditions, high price, etc. problems, to achieve the effects of easy industrial production, improved bioavailability, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 7.16 grams (0.02 moles) of Florfenicol, 6.07 grams (0.06 moles) of triethylamine, and 4 grams (0.04 moles) of succinic anhydride were added to a round-bottomed flask with a drying system filled with dichloromethane, Stir at 20°C, G254 thin-layer detection tracking (developing agent is chloroform:methanol:ethyl acetate:water=15:6:15:1 product Rf=0.32, florfenicol Rf=0.74), about 2 hours After the reaction was completed, the organic layer was washed with 10% hydrochloric acid, and then washed with water. The organic layer was dried over anhydrous sodium sulfate and concentrated to obtain 9 g of florfenicol succinate monoester with a yield of 98.3%.
[0018] 9 grams of the obtained florfenicol succinate monoester were dissolved in absolute ethanol, and reacted with sodium hydroxide solution at 10° C. to obtain 9.2 grams of florfenicol sodium succinate with a yield of 98%.
Embodiment 2
[0020] Add 7.16 grams (0.02 moles) of Florfenicol, 6.07 grams (0.06 moles) of triethylamine, and 4 grams (0.04 moles) of succinic anhydride into a round-bottomed flask with a drying system filled with chloroform, at 20°C Stir at low temperature for about 2 hours, wash the organic layer with 10% hydrochloric acid, wash with water, dry the organic layer with anhydrous sodium sulfate, and concentrate to obtain 8.5 g of florfenicol succinate monoester.
[0021] 8.5 grams of the obtained florfenicol succinate monoester was dissolved in absolute ethanol, and reacted with sodium hydroxide solution at 10° C. to obtain 8.7 grams of florfenicol succinate sodium with a yield of 98%.
Embodiment 3
[0023] 7.16 grams (0.02 moles) of Florfenicol, 6.07 grams (0.06 moles) of triethylamine, and 4 grams (0.04 moles) of succinic anhydride were added to a round-bottomed flask with a drying system filled with ethyl acetate, Stir at 20°C for about 2 hours, wash the organic layer with 10% hydrochloric acid, wash with water, dry the organic layer with anhydrous sodium sulfate, and concentrate to obtain 8.8 g of florfenicol succinate monoester.
[0024] 8.8 g of the obtained florfenicol succinate monoester was dissolved in absolute ethanol, and reacted with sodium hydroxide solution at 10° C. to obtain 9.0 g of florfenicol succinate sodium with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com