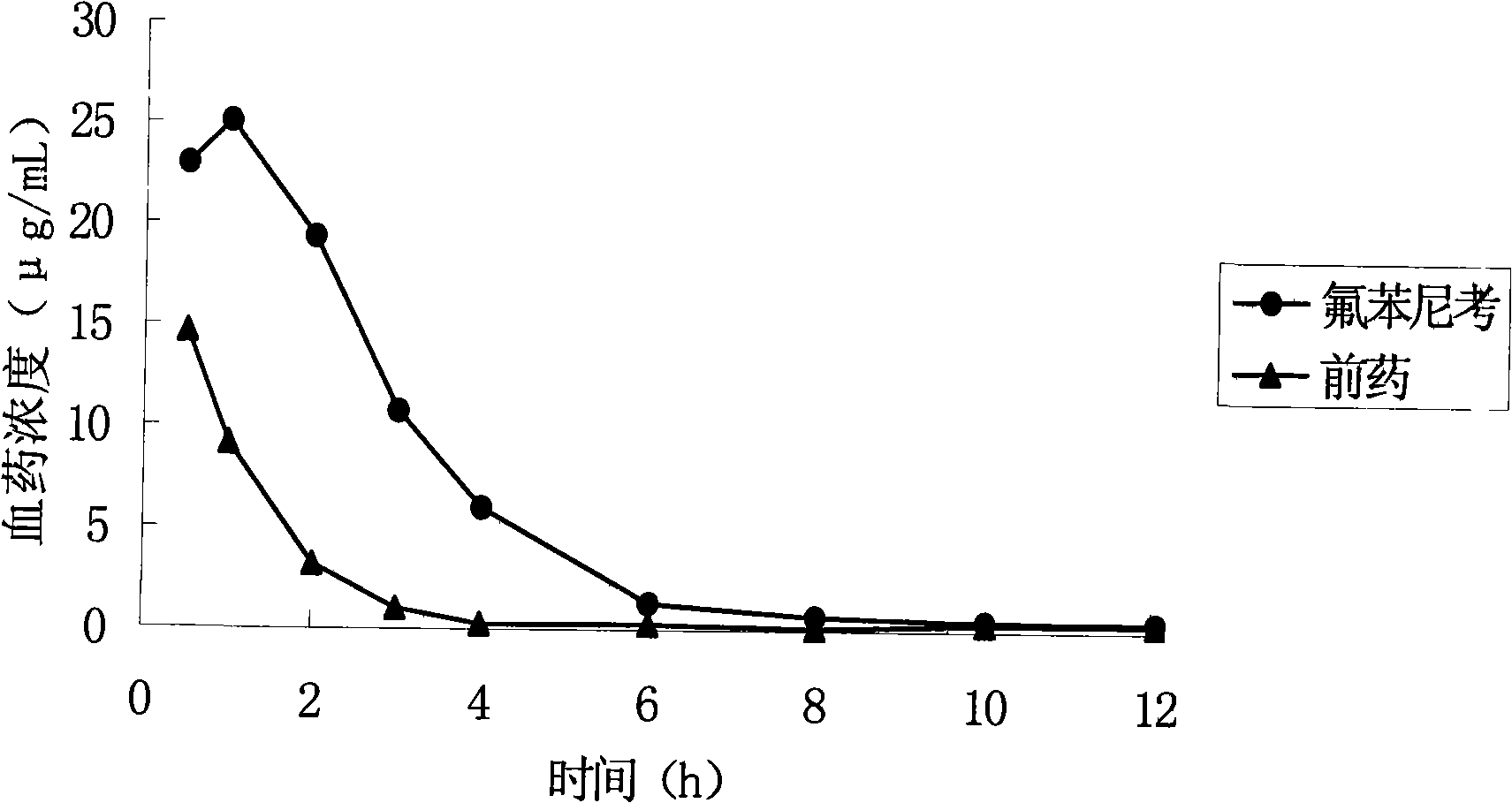
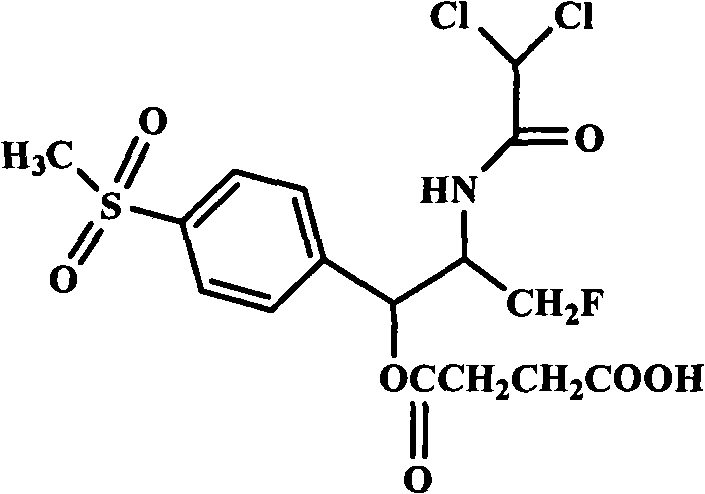
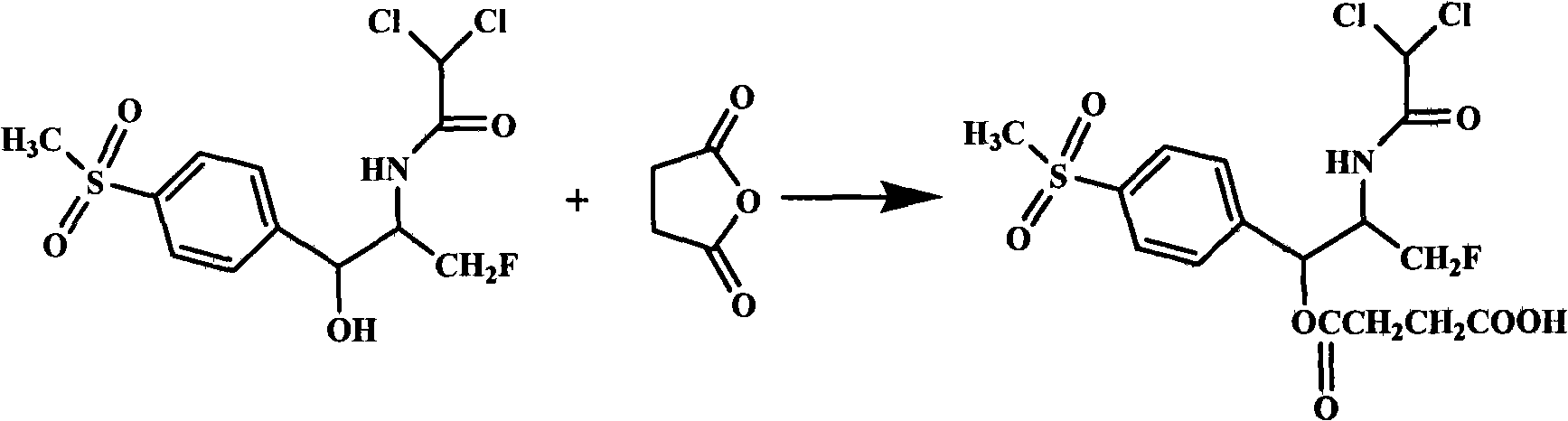
High-water-solubility florfenicol prodrug quickly released in vivo
A technology of florfenicol and florfenicol succinic acid, which is applied in the field of pharmaceutically acceptable salts of florfenicol succinic acid monoester and its preparation, and can solve the problems of difficult promotion, harsh synthesis conditions, cumbersome process, etc. problem, to achieve the effect of maintaining antibacterial activity, convenient medication, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 florfenicol succinic acid monoester
[0018] Under nitrogen flow, 10.0 g of florfenicol and 4.7 g of succinic anhydride were added to 50 mL of acetonitrile, and 1 mL of catalyst was added. Reflux at 80°C for 10 hours. Concentrate to dryness under reduced pressure, add 100 mL of ethyl acetate to dissolve, wash with dilute hydrochloric acid, wash with water, and evaporate to dryness under reduced pressure. The crude product was crystallized with ethanol, water, acetone, petroleum ether or their mixed solvents to obtain a solid, which was suction filtered and dried to obtain 9.7 g of white solid, mp 129.6-130.0°C, yield 75.8%.
[0019] IR (cm -1 ): 1672.95, 1702.21, 1741.50 (C=O);
[0020] 1 H-NMR (d 6 -DMSO, 400MHz) δ: 8.91 (1H, d), 7.60 ~ 7.89 (Ar-H, dd), 6.43 (1H, d), 6.01 (1H, d), 4.30 ~ 4.63 (3H, m), 3.19 ( 3H, s), 2.49 ~ 2.67 (4H, m);
[0021] 13 C-NMR (d 6 -DMSO, 400MHz) δ: 173.2, 171.1, 163.8, 126.8~142.6 (Ar-C), 82.7, 81.0,...
Embodiment 2
[0023] Embodiment 2 The preparation of florfenicol monosodium succinate (1)
[0024] Dissolve 0.84g of sodium bicarbonate in 10mL of water, and add 4.58g of florfenicol succinic acid monoester under stirring. When it is completely dissolved and no bubbles are released, add 5-15 times the amount of ethanol or acetone to form a precipitate. Suction filtration, vacuum drying of the solid at 90°C, yielded 3.96 g of florfenicol monosodium succinate, mp 157.4-158.3°C, yield 82.5%.
Embodiment 3
[0025] The preparation of embodiment 3 florfenicol succinate monoester sodium (2)
[0026] Dissolve 4.58g of florfenicol succinic acid monoester in 30mL of methanol, then add 0.53g of sodium carbonate and stir until no bubbles are released, put in activated carbon for decolorization and filtration, distill the filtrate under reduced pressure, crystallize the residue in 90% ethanol, and filter with suction The solid was dried to obtain 3.85 g of florfenicol monosodium succinate, with a yield of 80.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com