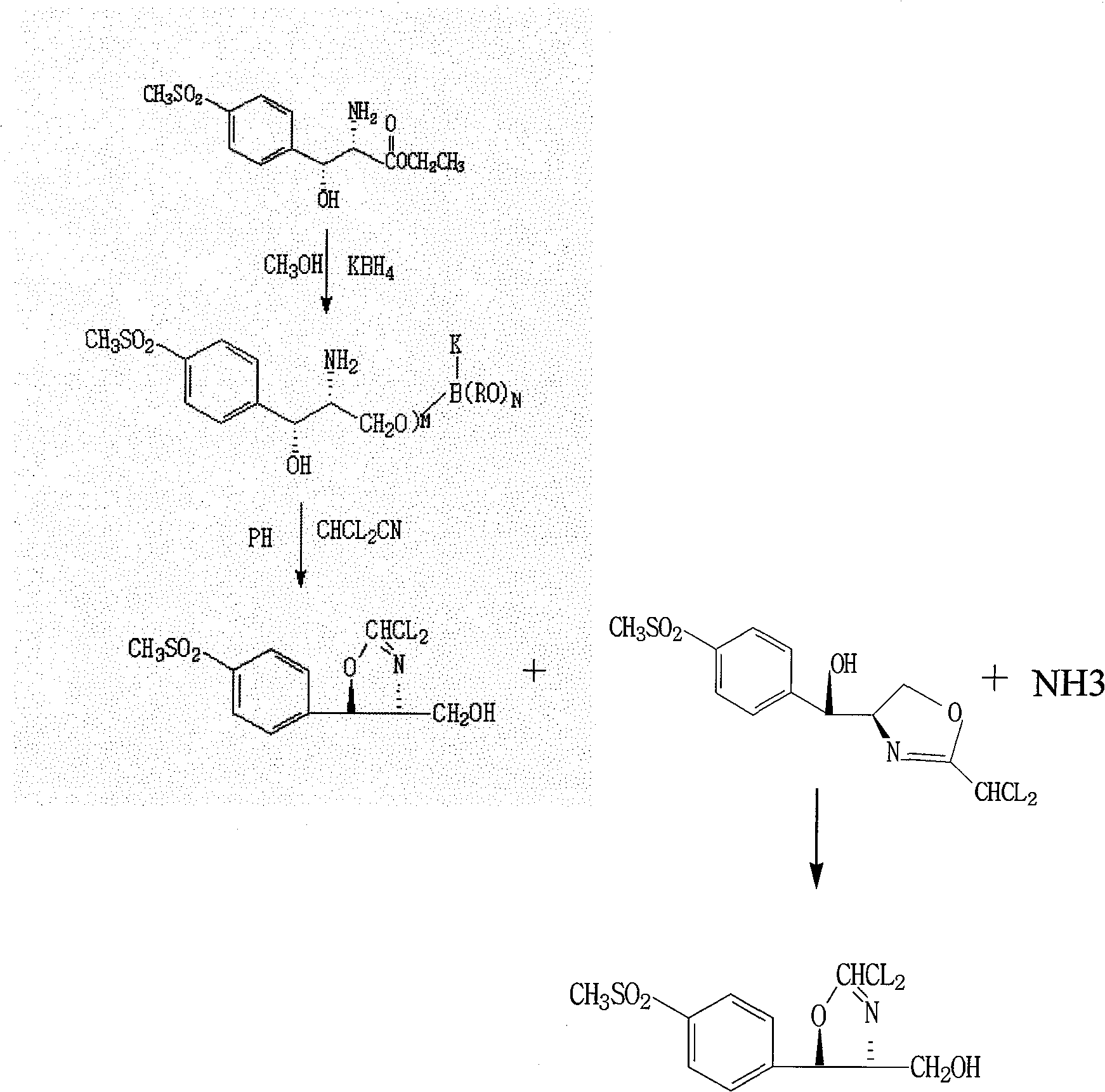
Preparation method of D-threo-2-(dichloromethyl)-4, 5-dihydro-5-(p-(methylsulfonyl) phenyl)-4-oxazole methanol
A technology of dichloromethyl and oxazole methanol, applied in the field of preparation of florfenicol intermediates, can solve the problems of high cost, long process steps, cumbersome operation, etc., and achieve low cost, simple operation and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
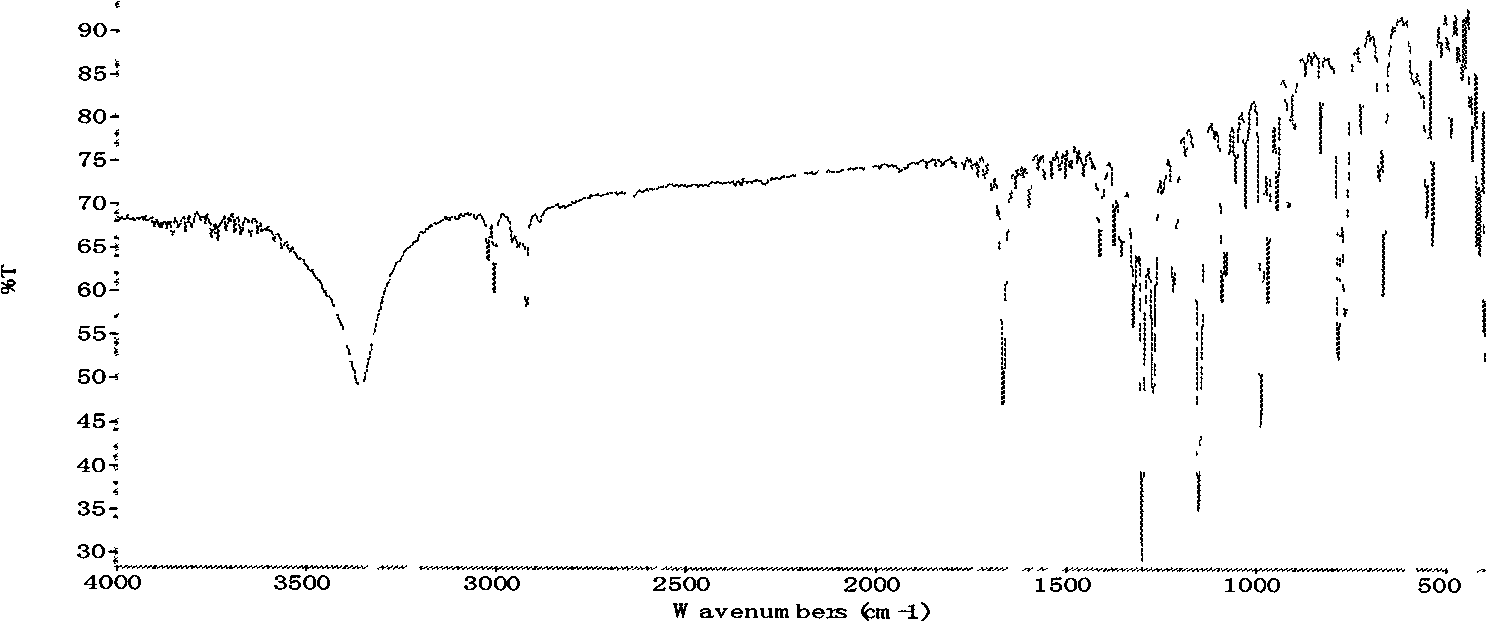
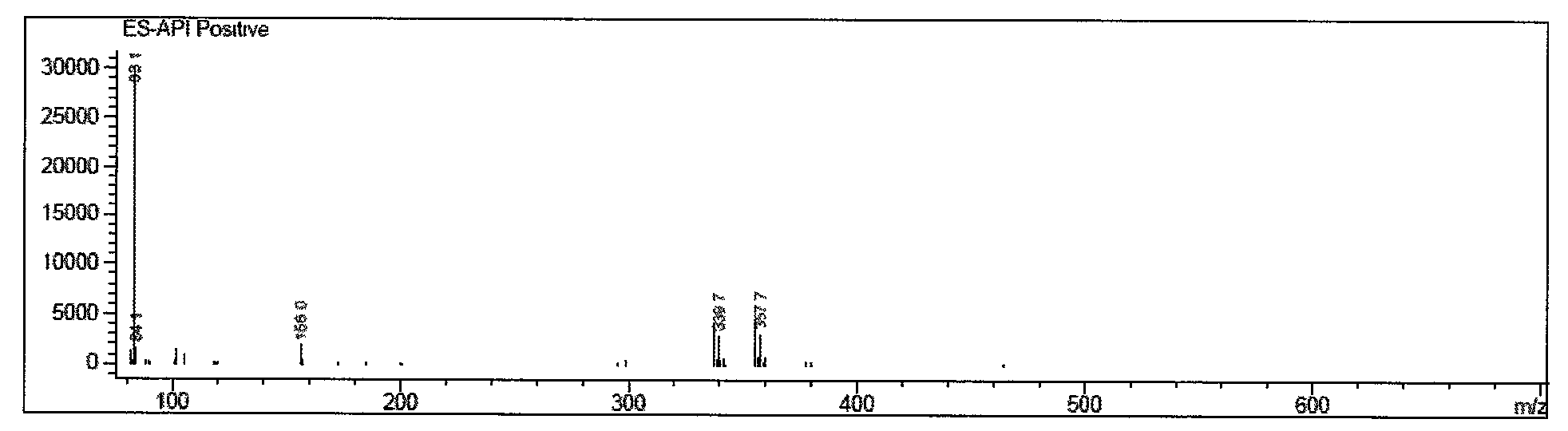
[0045] Add 100 g of D-p-thymphenylphenylserine ethyl ester (I) into 700 g of methanol solvent, add 27 g of potassium borohydride, and react at 30-50° C. After the reduction reaction is completed in about 6 hours. Distill under reduced pressure below 40°C, remove 600g of methanol, add 300g of glycerin to form a mixed solvent, add hydrochloric acid to neutralize, adjust pH = 7.5, add 44g of dichloroacetonitrile, stir at 50°C for 18 hours, recover methanol by distillation under reduced pressure below 40°C , add isopropanol aqueous solution, stir, filter, and dry to obtain D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(thysulfonyl)phenyl]- 103 g of 4-oxazole methanol (III). Melting point: 143-144°C; Optical rotation: [a] 20 D +11.5, IR KBr cm-1:: 3350, 2930, 1670; ESI-MS (M+1): 339.7; hydrogen spectrum data, carbon spectrum data are shown in Table 2, yield 103%.
[0046] Table 2
[0047]
[0048] Literature sources of reference compounds: Guangzhong Wu, Doris P.Schumacher, ...
Embodiment 2
[0050] Add 100 g of D-p-thymphenylphenylserine ethyl ester (I) into 700 g of methanol solvent, add 27 g of potassium borohydride, and react at 30-50° C. After the reduction reaction is completed in about 6 hours. Distill under reduced pressure below 40°C, remove 600g of methanol, add 300g of glycerin to form a mixed solvent, add sulfuric acid to neutralize, adjust pH = 10.0, add 44g of dichloroacetonitrile, stir at 50°C for 18 hours, and recover methanol by distillation under reduced pressure below 40°C , add isopropanol aqueous solution, stir, filter, and dry to obtain D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(thysulfonyl)phenyl]- 82 g of 4-oxazole methanol (III), the yield was 82%.
Embodiment 3
[0052] Add 100 g of D-p-thymphenylphenylserine ethyl ester (I) into 700 g of methanol solvent, add 27 g of potassium borohydride, and react at 30-50° C., after 6 hours of reduction reaction. Distill under reduced pressure below 40°C, remove 600g of methanol, add 300g of glycerin to form a mixed solvent, add acetic acid to neutralize, adjust pH=7.1, add 44g of dichloroacetonitrile, stir at 50°C for 18 hours, and recover methanol by distillation under reduced pressure below 40°C , add isopropanol aqueous solution, stir, filter, and dry to obtain D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(thysulfonyl)phenyl]- 99g of 4-oxazolemethanol (III), the yield is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com