Method for synthesizing florfenicol
A technology of florfenicol and synthetic method, which is applied in the field of florfenicol synthesis, can solve the problems of low conversion rate, many by-products, rising synthesis cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
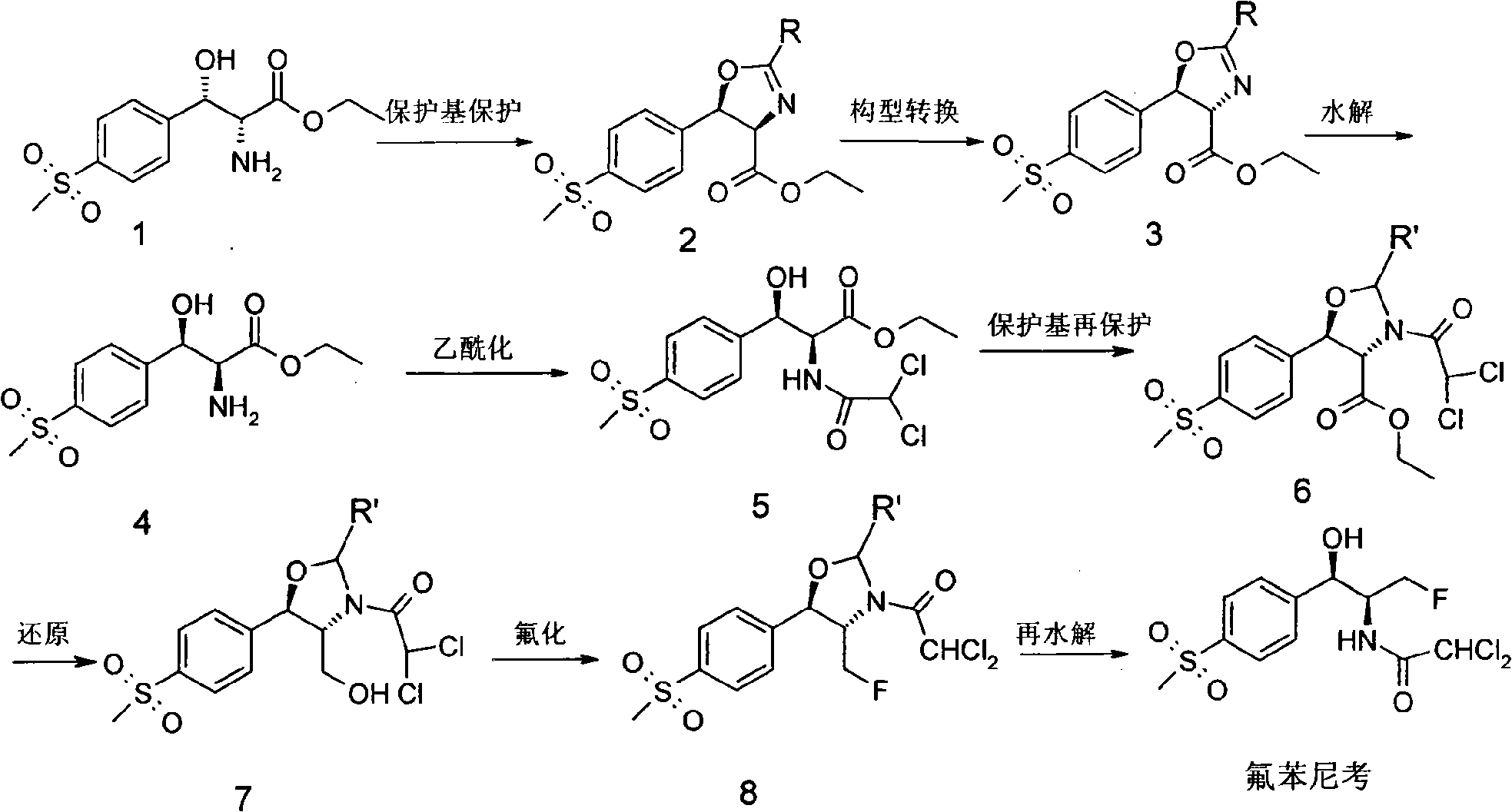
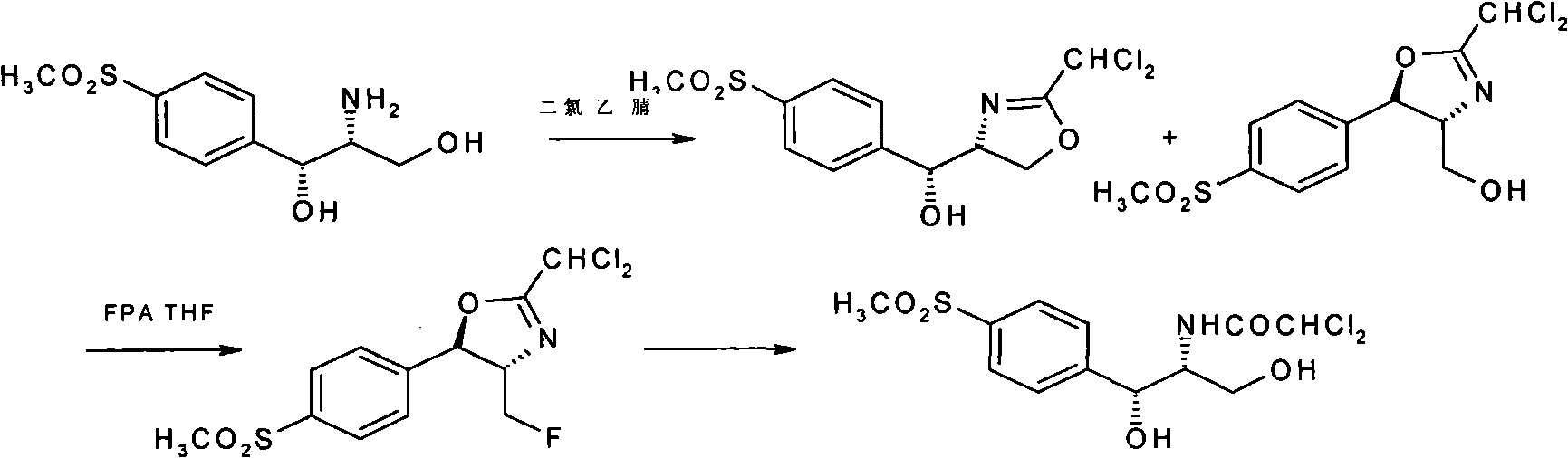
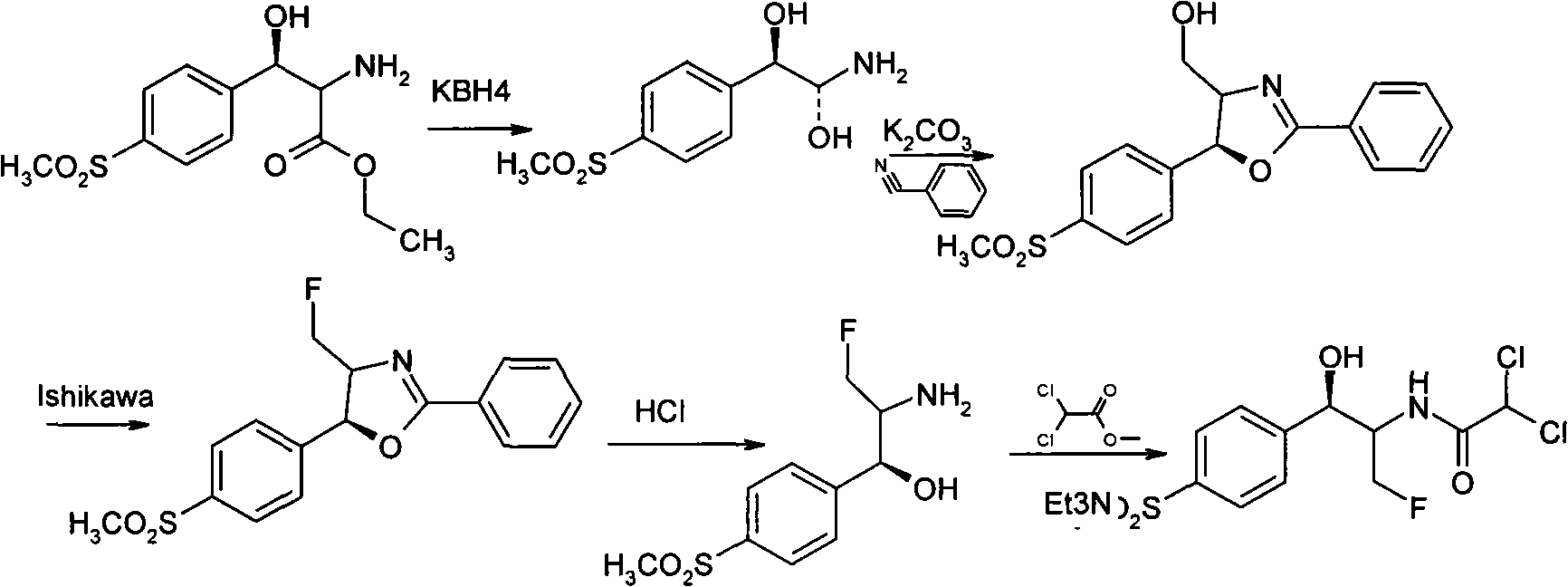
[0058] How to implement the present invention will be further described below in conjunction with specific examples. The following examples are helpful for understanding the present invention, but are not limited to the content of the present invention.
[0059] (A) compound 1 is protected by protecting group to prepare compound 2
[0060] Dissolve 5 g of L-threo-[p-(thysulfonyl)phenyl]serine ethyl ester in 10 ml of methanol and 30 ml of dichloromethane, and cool to 10° C. in an ice bath. Mix benzoyl chloride with 2.2ml of dichloromethane and add it dropwise into the reaction flask, control the temperature not higher than 20°C, stir at room temperature for 2 hours, add 2.7g of thionyl chloride, and react compound 1 with benzoyl chloride The reaction was carried out at a ratio of 1:1.5. The reaction system was heated to 50° C. and kept at this temperature for 2 hours, then naturally cooled to room temperature, added 30 g of ice water, stirred for 30 minutes, and stood to separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com