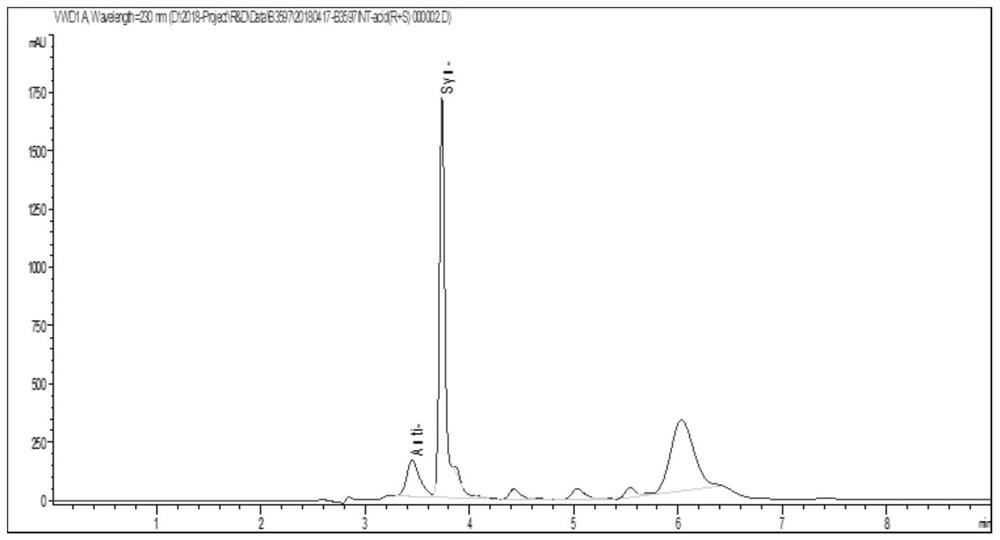
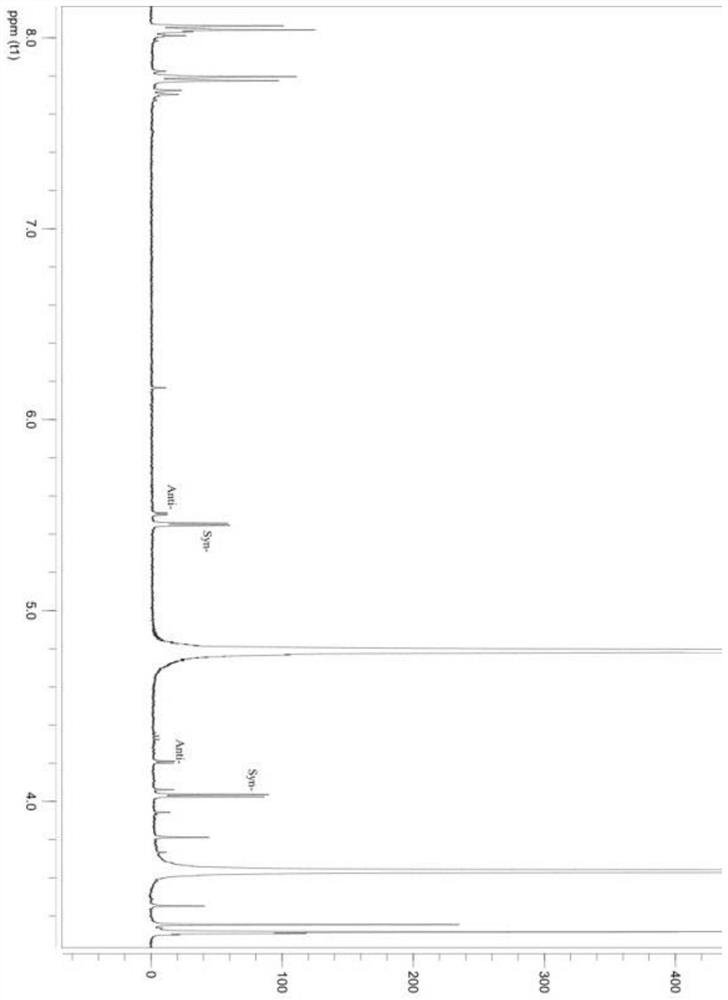
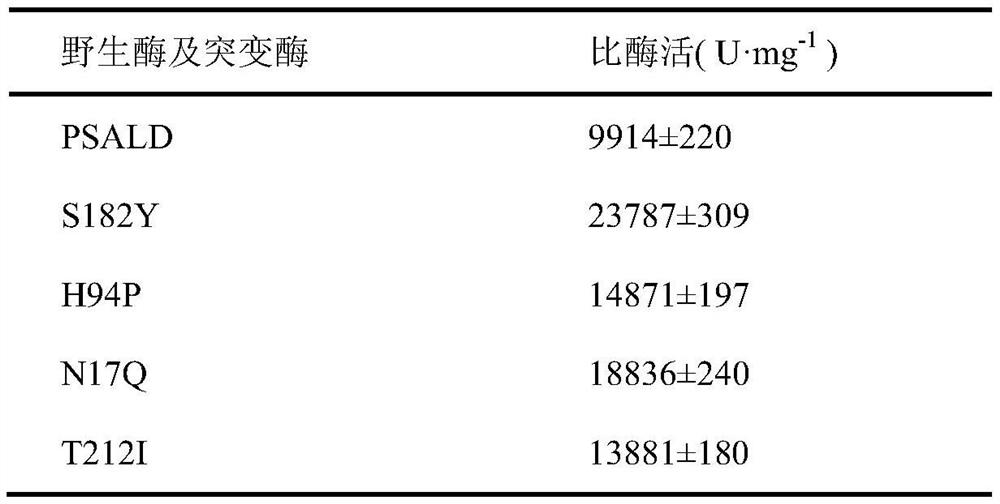
Threonine aldolase, mutants and their application in the preparation of substituted phenylserine derivatives
A technology of threonine aldolase and phenylserine, applied in the application field of phenylserine derivatives, can solve the problem of insufficient chiral selectivity, limited application, and poor stability of threonine aldolase And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Amplification of threonine aldolase gene PSALD
[0026] According to the threonine aldolase gene information from Pseudomonas putida included in Genebank (the gene sequence is shown in SEQ ID NO: 1), the total genomic DNA of the thalline was extracted with a rapid nucleic acid extraction instrument, Using the genomic DNA as a template, PCR amplification was performed under the action of primer 1 (ATGAATGGTGAAA CCAGCCG) and primer 2 (TTAACGTTCCTGGGTGCGAT). PCR reaction system (total volume 50 μL): 5 μL of 10×Pfu DNA Polymerase Buffer, 1 μL of 10 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), 1 μL of cloning primer 1 and primer 2 each at a concentration of 50 μM, 1 μL of genomic DNA, Pfu DNA Polymerase 1μL, nucleic acid-free water 40μL.
[0027] Using BioRad PCR instrument, PCR reaction conditions: pre-denaturation at 95°C for 5min, denaturation at 95°C for 30s, tempering at 65°C for 45s, extension at 72°C for 1min, a total of 30 cycles, and fina...
Embodiment 2
[0028] Example 2: Amplification of Threonine Aldolase Gene CCALD
[0029] According to the threonine aldolase gene information from Caulobacter crescentus included in Genebank (the gene sequence is shown in SEQ ID NO: 88), the total genomic DNA of the thalline was extracted with a rapid nucleic acid extraction instrument, Using the genomic DNA as a template, PCR amplification was performed under the action of primer 3 (ATGACCCAFACCGCGCCCCGCTA) and primer 4 (CTAAGCCACTCGCTTCAGCGCC). PCR reaction system (total volume 50 μL): 5 μL of 10×PfuDNA Polymerase Buffer, 1 μL of 10 mM dNTPmixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), 1 μL of cloning primer 1 and primer 2 each at a concentration of 50 μM, 1 μL of genomic DNA, Pfu DNA Polymerase 1μL, nucleic acid-free water 40μL.
[0030] Using BioRad PCR instrument, PCR reaction conditions: pre-denaturation at 95°C for 5min, denaturation at 95°C for 30s, tempering at 65°C for 45s, extension at 72°C for 1min, a total of 30 cycles, a...
Embodiment 3
[0031] Embodiment 3: the construction of the recombinant escherichia coli of threonine aldolase gene PSALD
[0032] 1. Obtaining the recombinant plasmid pET28a-PSALD
[0033] Obtain the PSALD gene sequence, use Nde I and Xho I restriction endonuclease (TaKaRa) to process the amplified fragment after sequencing, and use T 4 DNA ligase (TaKaRa) ligated the fragment with the commercialized vector pET28a treated with the same restriction endonuclease to construct the expression vector pET28a-PSALD.
[0034] 2. Transformation of Escherichia coli E.coli BL21(DE3) with recombinant plasmid
[0035] Add 10 μL of PCR product to 100 μL E.coli BL21 (DE3) competent cell suspension in each tube, mix gently, and let stand in ice bath for 30 min. Transfer to a 42°C water bath and heat shock for 90s. Quickly transfer to an ice bath to cool for 3 min. Add 700 μL LB liquid medium to each tube, and incubate at 37° C. for 1 hour on a shaker at 100 rpm. After culturing, the bacterial solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com