Method for synthesizing noradrenaline through double-enzyme coupling
A technology of norepinephrine and crude enzyme solution, which is applied in the field of double-enzyme coupling synthesis of norepinephrine, which can solve the problems of many by-products and high cost of disassembly, and achieve the effects of low production cost, environmental friendliness and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1, construct the genetically engineered bacteria BL21(DE3) / pGEX-6p-1-S-TA expressing S-TA
[0036] After codon optimization based on the reported amino acid sequence of L-threonine aldolase from black bears, the sequence is shown in SEQ NO.1, and the optimized sequence was synthesized by entrusting Qingke Biotechnology Co., Ltd. (Nanjing), Subcloned to the vector pGEX-6p-1 to obtain the recombinant plasmid pGEX-6p-1-S-TA, and transformed the constructed recombinant plasmid pGEX-6p-1-S-TA into the E. coli expression host by the calcium chloride method BL21(DE3), the strain BL21(DE3) / pGEX-6p-1-S-TA expressing L-threonine aldolase was obtained;
[0037] Step 2, construct the genetically engineered bacteria BL21(DE3) / pCDF-duet-TDC expressing TDC
[0038] After codon optimization based on the reported amino acid sequence of tyrosine decarboxylase derived from Lactobacillus brevis, the sequence is shown as SEQ NO.2, and the optimized sequence was entrusted to Qingke Bio...
Embodiment 2
[0048] To catalyze the production of norepinephrine, the specific operation steps are as follows: the reaction system is 1ml, the final concentration of 3,4-dihydroxybenzaldehyde is 60mM / L, the final concentration of glycine is 1M / L, and the final concentration of pyridoxal 5-phosphate is 20μm / L, then add 1g / L S-TA, 6g / L TDC, adjust the system to 1ml with PBS buffer, pH 7.5, and react at 30°C for 2-12h.
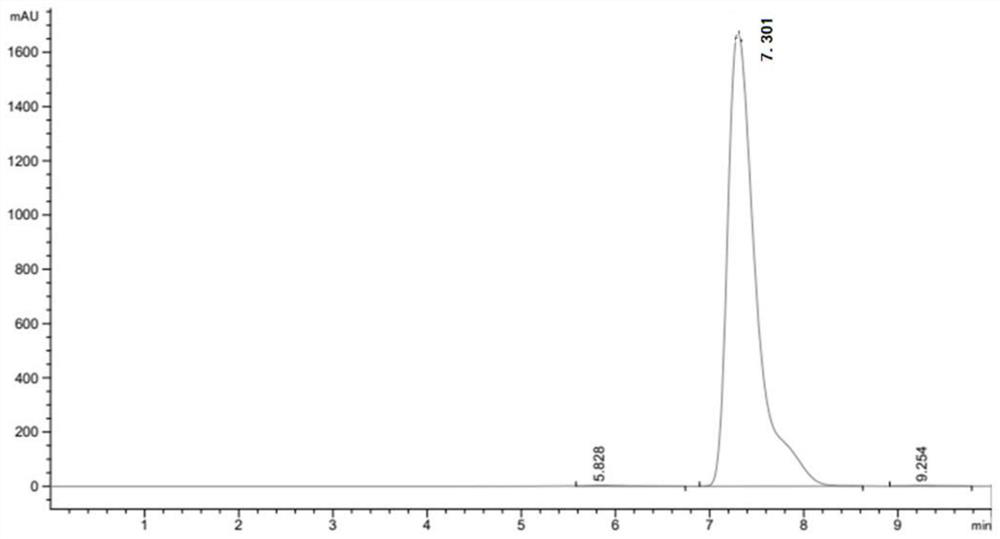
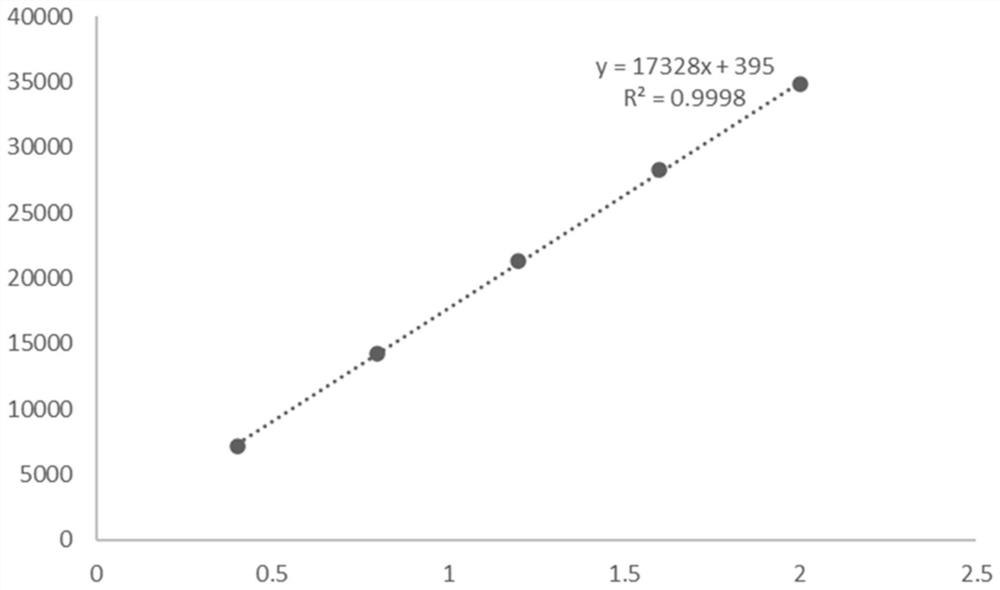
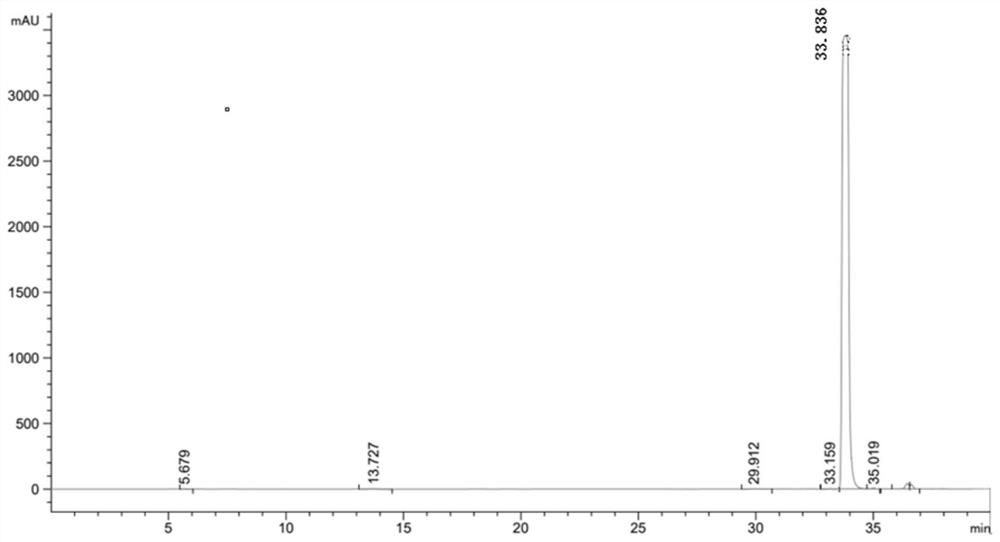
[0049] After the reaction, utilize high-performance liquid chromatography, XSelect HSS T3 type chromatographic column (4.6 * 250mm, 5 μ m), mobile phase is 23.3g sodium chloride (Shanghai Merrill Chemical Technology Co., Ltd.), 1ml glacial acetic acid, dilute with water to 1 L, mixed with 2% pure acetonitrile (analytical grade) solution, the column temperature was 30°C, the ultraviolet detection wavelength was 280 nm, and the flow rate was 5ml / min.
[0050] Take the gradient elution method:
[0051]
[0052] After detection by high performance liquid chromatography, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com