Method for producing L-2-aminobutyric acid by virtue of biological catalysis
An aminobutyric acid and amino acid technology, applied in the field of biosynthesis, can solve the problems of difficult separation and purification, harsh reaction conditions, cumbersome processes, etc., and achieve the effects of improving chiral purity, low equipment requirements, and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
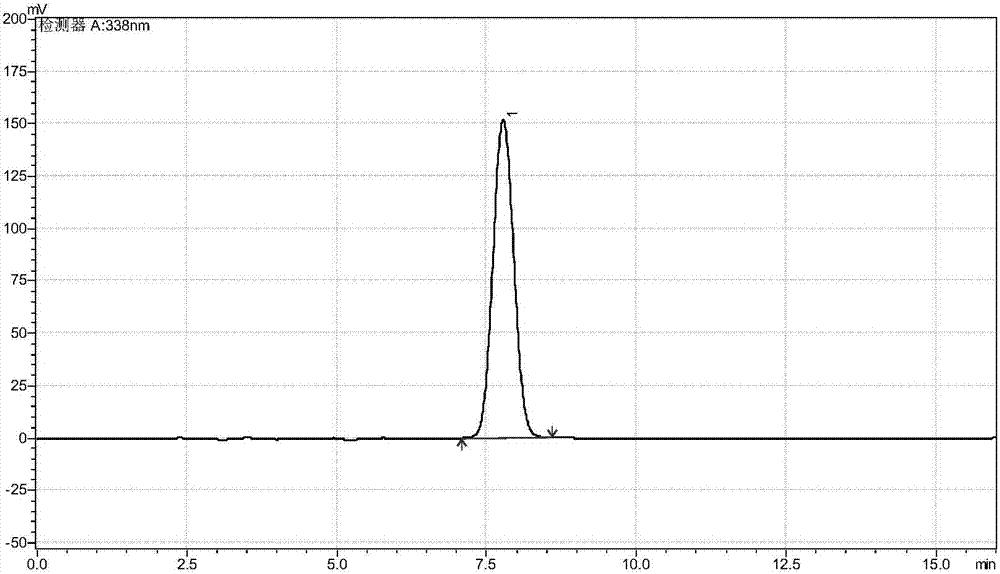
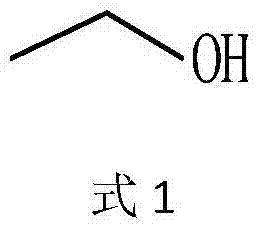
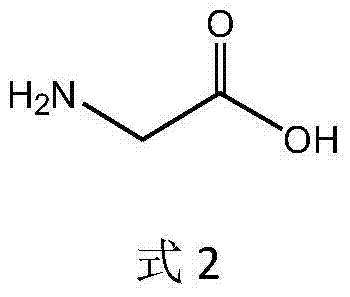
[0041]Take 75.0g of anhydrous glycine and add an appropriate amount of deionized water to dissolve it, then add 120mL of absolute ethanol solution and dissolve it with 3.0mol / L ammonia water, adjust the pH to 8.0±0.05 with hydrochloric acid, remove insoluble matter by vacuum filtration, and set the volume to 1000mL. Add liquid alcohol dehydrogenase 4500U, immobilized threonine aldolase 3000U, immobilized threonine deaminase 6000U and immobilized L-amino acid dehydrogenase 4500U, NAD28.0mg, pyridoxal phosphate 15.0mg, control The reaction temperature is 35°C, and the pH is maintained at 8.00±0.05 with 3.0 mol / L ammonia water. When the molar conversion rate of glycine detected by the liquid phase is ≥99%, the conversion termination solution is filtered out. The stop solution was adjusted to pH 6.0±0.05 with 5.0 mol / L formic acid, and then electrodialysis was performed to desalt and remove water to obtain 1530 mL of a solution containing 92.04 g of L-α-aminobutyric acid, with a yi...
example 2
[0043] Take 75.0g of anhydrous glycine and add an appropriate amount of deionized water to dissolve it, then add 120mL of absolute ethanol solution and dissolve it with 3.0mol / L ammonia water, adjust the pH to 8.0±0.05 with hydrochloric acid, remove insoluble matter by vacuum filtration, and set the volume to 1000mL. Add liquid alcohol dehydrogenase 25000U, immobilized threonine aldolase 5000, immobilized threonine deaminase 25000 and immobilized L-amino acid dehydrogenase 20000U, NAD28.0mg, pyridoxal phosphate 15.0mg, control The reaction temperature is 35°C, and the pH is maintained at 8.00±0.05 with 3.0 mol / L ammonia water. When the molar conversion rate of glycine detected by the liquid phase is ≥99%, the conversion termination solution is filtered out. The stop solution was adjusted to pH 6.0±0.05 with 5.0 mol / L formic acid, and then electrodialysis was performed to desalt and remove water to obtain 1530 mL of a solution containing 95.04 g of L-α-aminobutyric acid, with a ...
example 3
[0045] Take 75.0g of anhydrous glycine and add an appropriate amount of deionized water to dissolve it, then add 120mL of absolute ethanol solution and dissolve it with 3.0mol / L ammonia water, adjust the pH to 8.0±0.05 with hydrochloric acid, remove insoluble matter by vacuum filtration, and set the volume to 1000mL. Add liquid alcohol dehydrogenase 8000U, immobilized threonine aldolase 4000U, immobilized threonine deaminase 12000U / mol and immobilized L-amino acid dehydrogenase 8000U, NAD28.0mg, pyridoxal phosphate 15.0mg , control the reaction temperature at 35° C., use 3.0 mol / L ammonia water to maintain the pH at 8.00±0.05, and filter out the conversion termination solution when the molar conversion rate of glycine is ≥99% as detected by the liquid phase. The stop solution was adjusted to pH 6.0±0.05 with 5.0 mol / L formic acid, and then electrodialysis was performed to desalt and remove water to obtain 1530 mL of a solution containing 95.44 g of L-α-aminobutyric acid, with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com