High-temperature-resistant L-threonine aldolase and application thereof to synthesis of p-methylsulfonyl phenyl serine
A technology of thiamphenylphenylserine and threonine aldolase, which is applied in the field of enzyme engineering, can solve problems such as difficult synthesis, and achieve the effects of simple steps, mild reaction conditions and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the whole cell of above-mentioned recombinant escherichia coli comprises the following steps:
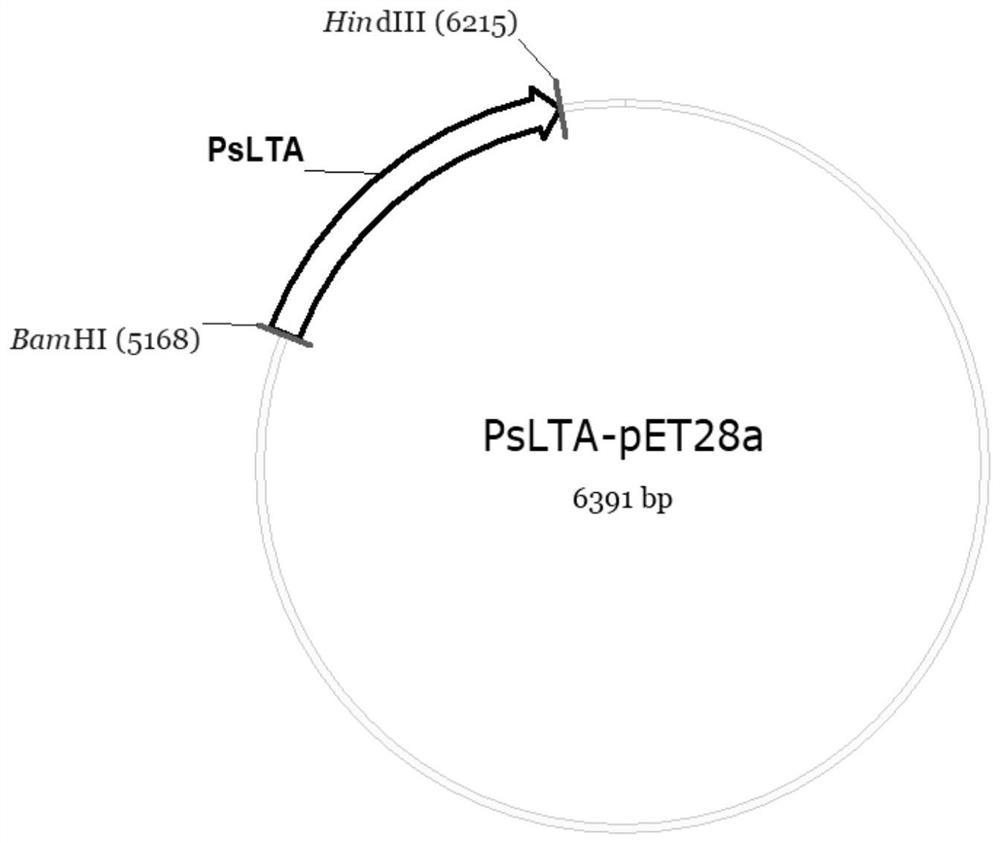
[0039] S1: Cloning the target gene having the nucleotide sequence shown in SEQ ID NO: 1 into the restriction site between BamHI and HindIII of the vector pET-28a to construct the recombinant expression plasmid vector PsLTA-pET-28a;
[0040] S2: Transform the recombinant expression plasmid vector PsLTA-pET-28a described in S1 into Escherichia coli BL21 competent cells, and cultivate the recombinant strain E.coli BL21 / PsLTA-pET-28a;
[0041] S3: Collect the recombinant E. coli BL21 / PsLTA-pET-28a cultured in S2, and obtain the whole cells of the recombinant E. coli.
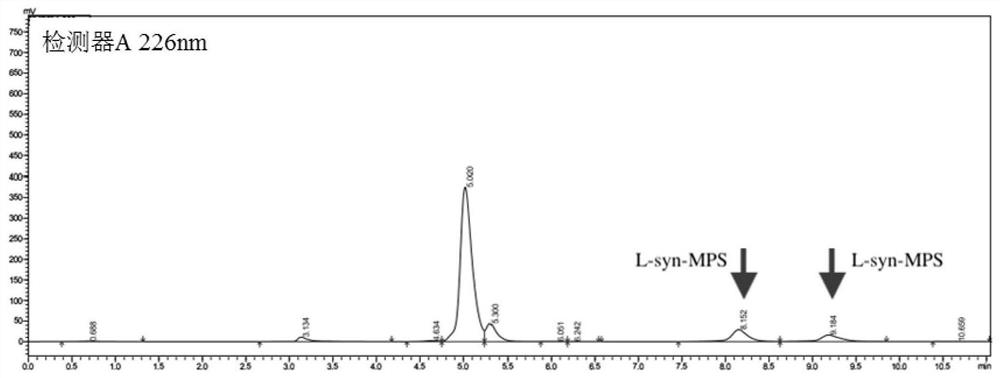
[0042] The application of the above-mentioned L-threonine aldolase in the synthesis of p-thymphenylphenylserine.
[0043] Preferably, the whole cell of recombinant Escherichia coli containing the nucleotide sequence shown in SEQ ID NO:1 is used as the catalyst, and the L-threonine aldolas...
Embodiment 1
[0046] Example 1: Construction of recombinant expression plasmid vector PsLTA-pET-28a
[0047] The construction of the plasmid was codon-optimized by Nanjing GenScript Co., Ltd. from the LTA gene (nucleotide sequence shown in SEQ ID NO: 1) derived from Pelosinus sp., and then cloned into the BamHI and HindIII of the vector pET-28a Between the restriction enzyme sites, the recombinant expression plasmid vector PsLTA-pET-28a was constructed ( figure 1 ).
Embodiment 2
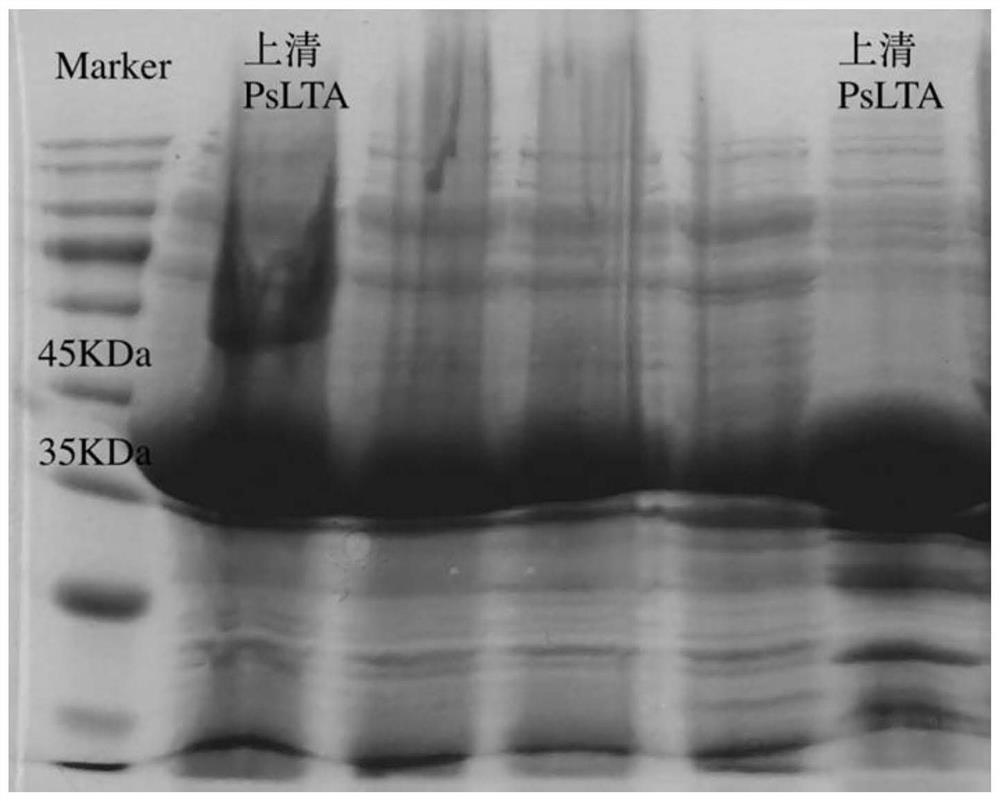
[0048] Embodiment 2: Construction and cultivation of recombinant bacteria E.coli BL21 / PsLTA-pET-28a The plasmid carrier PsLTA-pET-28a constructed by the present invention is transformed into competent cells of E.coli DH5α for cloning, and then the plasmid is extracted and transformed into In E.coliBL21 competent cells, an expression strain was constructed for protein expression. Pick a single colony from the plate and inoculate it into a test tube of LB liquid medium containing 50 μg / mL kanamycin. After cultivating at a constant temperature and shaking at 37°C and 220 rpm for about 12 hours, inoculate the tube containing 50 μg / mL kanamycin according to 1% inoculation amount. Namycin in TB liquid medium. Determination of OD of culture medium by ultraviolet spectrophotometry 600 When reaching 0.6-0.8, add 0.2mM inducer IPTG to induce protein expression. The expression was induced for 22 hours at 16°C and 220rpm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com