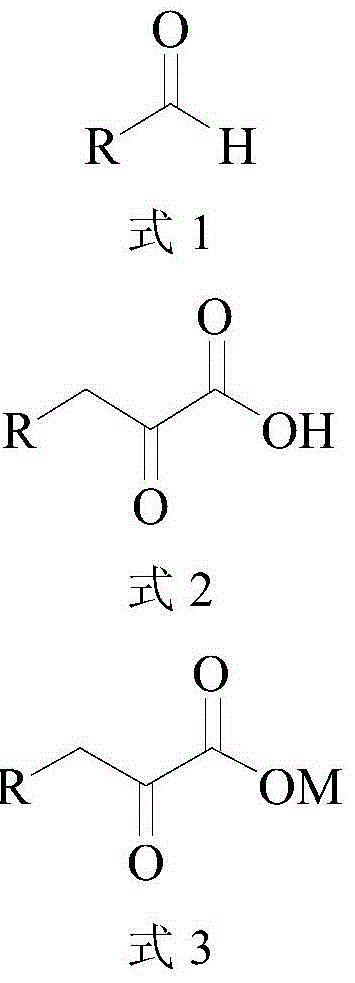
Method for synthesizing high-purity 2-ketonate
A keto acid salt, high-purity technology, applied in the separation/purification of carboxylic acid compounds, organic chemistry, fermentation and other directions, can solve the problems of cumbersome chemical treatment steps, large amount of organic solvent, unfavorable industrial production, etc. The effect of mild conditions, lower production costs, and environmental protection in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
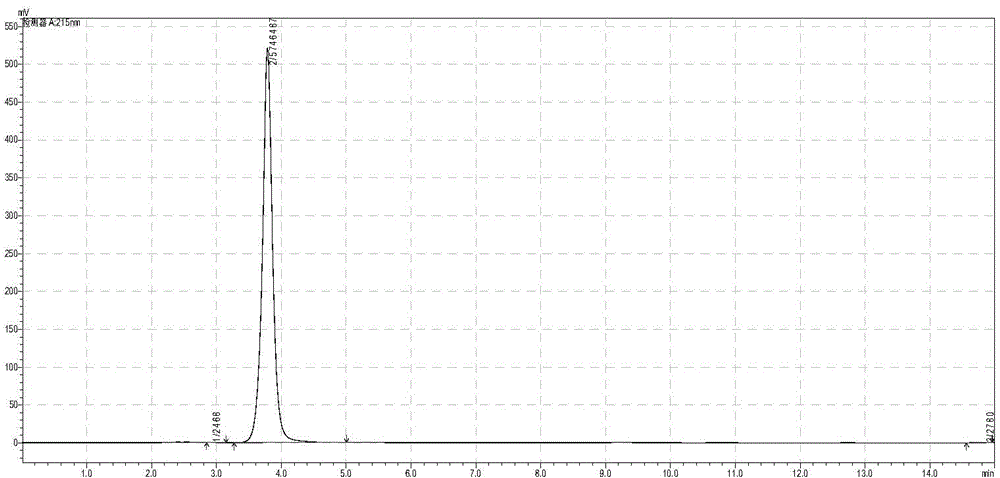
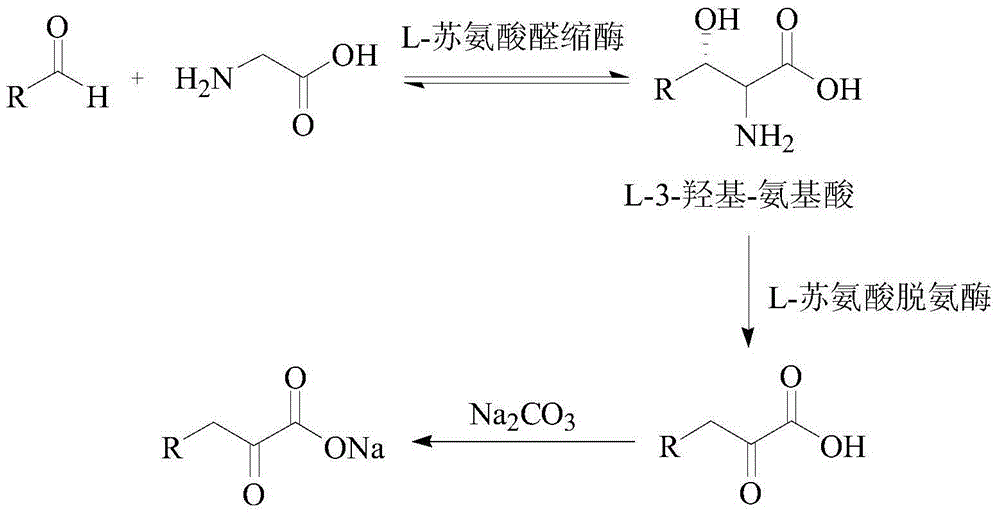
[0033] Take 35.7 grams of glycine (anhydrous) and add an appropriate amount of deionized water to dissolve it, add 40% 45.74mL of acetaldehyde solution, dissolve it with 3.0mol / L sodium hydroxide solution and control the pH at about 7.50, and vacuum filter to remove the insoluble and dilute to 600mL. Input L-threonine aldolase by 30000U / L (wherein U is the total enzyme activity of enzyme input, L is the reaction volume) ratio and by 20000U / L (wherein U is the total enzyme activity of enzyme input, L is Reaction volume) ratio into L-threonine deaminase, control reaction temperature 30 ℃, keep pH at 7.50 with 3.0mol / L sodium hydroxide solution in the reaction process, stop reaction when liquid phase glycine remains below 0.2%, The transformation stop solution was filtered off. 624 mL of a solution containing 45.80 g of 2-butanuonic acid was obtained, and the yield was 98.0%. Put the conversion termination liquid directly on the 200mL ion exchange chromatography column regenera...
Embodiment 2
[0048] Take 37.8 grams (anhydrous) glycine and add appropriate amount of deionized water to dissolve it, then add 97% 35.6mL propionaldehyde solution, dissolve it with 3.0mol / L sodium hydroxide solution and control the pH at about 7.50, vacuum filter to remove insoluble matter , and set the volume to 600mL. Add 30000U / L of L-threonine aldolase and 20000U / L of L-threonine deaminase, control the reaction temperature at 30°C, and keep the pH at 7.50±0.05 with 3.0mol / L sodium hydroxide. When the conversion rate was greater than or equal to 99%, the conversion-terminated solution was filtered out to obtain 626 mL of a solution containing 54.73 g of 2-pentanuonic acid, with a yield of 98.3%. Put the conversion termination liquid directly on the 200mL ion exchange chromatography column regenerated by hydrochloric acid, the flow rate is: 6.67mL / min (2.0Bv / h). Directly use deionized water as the eluent to collect 2-pentanuonic acid, the range of collected liquid is 120-815 mL, the vol...
Embodiment 3
[0050] Take 34.0 grams of glycine (anhydrous) and add an appropriate amount of deionized water to dissolve it, then add 40% 52.4 mL of acetaldehyde solution, dissolve it with 3.0 mol / L sodium hydroxide solution and control the pH at about 7.50, and vacuum filter to remove the insoluble and dilute to 600mL. Add L-threonine aldolase at a ratio of 40000U / L and L-threonine deaminase at a ratio of 20000U / L, control the reaction temperature at 30°C, and use 3.0mol / l sodium hydroxide solution during the reaction Keep the pH at 7.50, terminate the reaction when the glycine in the liquid phase remains below 0.2%, and filter out the conversion termination solution. 644 mL of a solution containing 44.08 g of 2-butanuonic acid was obtained, and the yield was 94.3%. Put the conversion termination liquid directly on the 200mL ion exchange chromatography column regenerated by hydrochloric acid, the flow rate is: 6.67mL / min (2.0Bv / h). Directly use deionized water as the eluent to collect 2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com