Engineering bacterium for co-expressing L-threonine aldolase and PLP synthase and application
A technology of threonine aldolase and engineering bacteria is applied to engineering bacteria and application fields that co-express L-threonine aldolase and PLP synthase, and can solve the problem of large amount of coenzyme PLP and low utilization rate of free enzyme, etc. problem, to achieve the effect of low cost, high reuse efficiency and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: construction of engineering bacteria
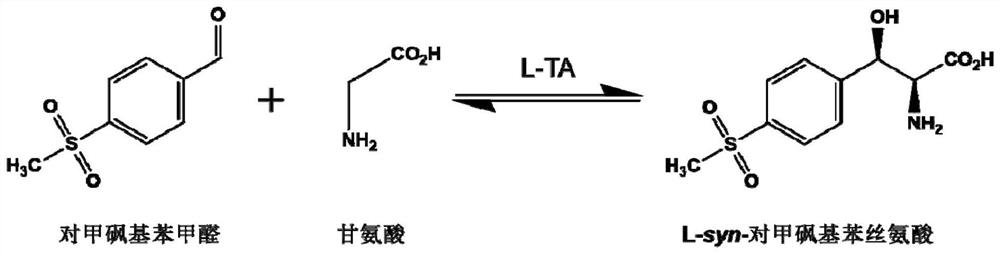
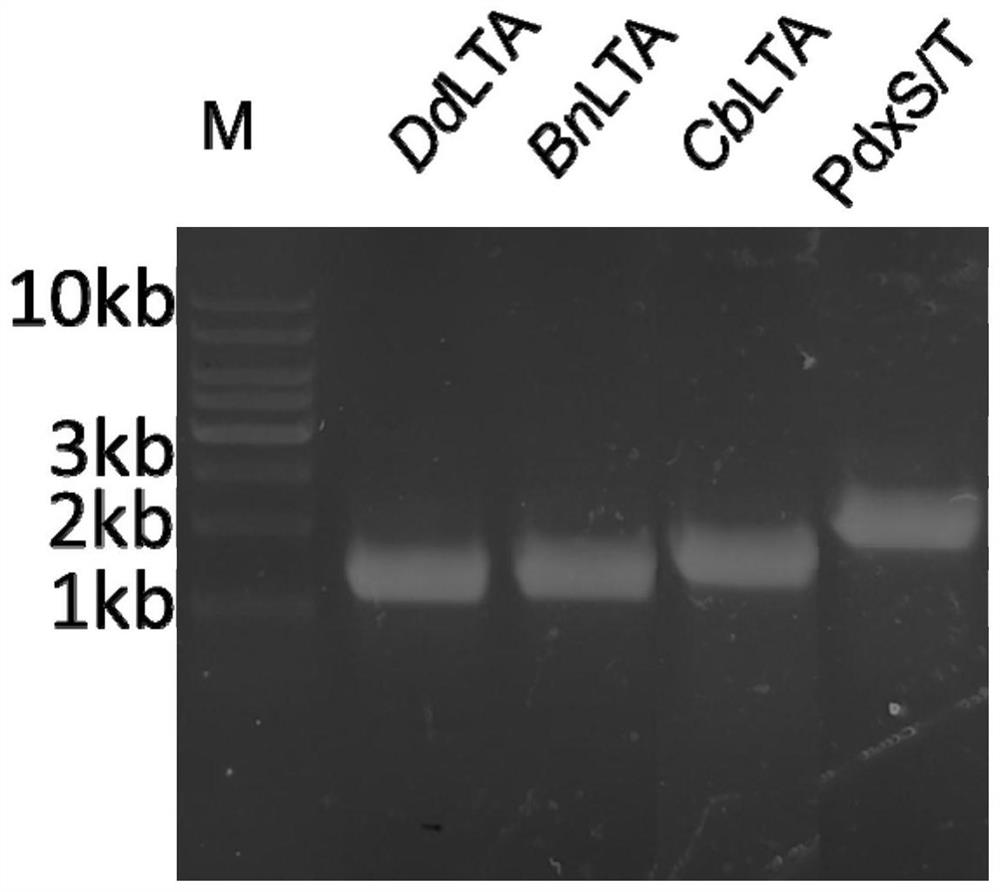
[0035] The NCBI accession number of L-threonine aldolase (hereinafter referred to as DdLTA) derived from Desulfitobacterium dichloroeliminans is WP_015261381.1. The NCBI accession number of L-threonine aldolase (hereinafter referred to as BnLTA) derived from Bacillus nealsonii is WP_016204489.1. The NCBI accession number of L-threonine aldolase (hereinafter referred to as CbLTA) derived from Clostridium beijerinckii is WP_023973138.1. The above three enzymes were optimized according to the gene sequence in Escherichia coli to obtain the gene sequences shown in SEQ ID No.1, SEQ ID No.2 or SEQ ID No.3 respectively.
[0036] The PLP synthase (hereinafter referred to as ST) derived from Bacillus subtilis has a heteromultimer structure, and the NCBI accession numbers of the two subunits are QJR44475.1 and QJR44476.1 respectively. The gene sequences of the two subunits are based on the After the gene sequence is optimized...
Embodiment 2
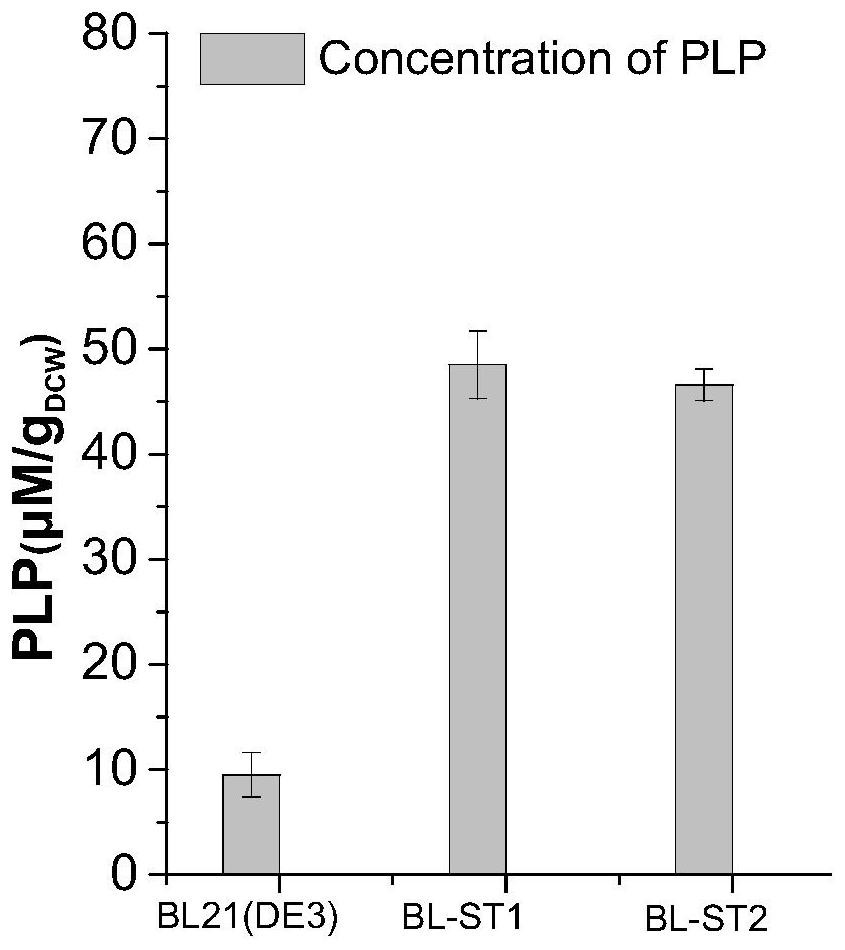
[0057] Embodiment 2: the preparation of the culture of thalline and crude enzyme liquid
[0058] 1. Bacteria culture
[0059] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 115°C for 30min, ready for use.
[0060] Streak the co-expression engineered bacteria on a plate, activate at 37°C for 12h, pick a single colony and inoculate into 5mL LB liquid medium containing the corresponding antibiotic (50μg / mL), and culture with shaking at 37°C for 8-10h. Transfer 1% of the inoculum into 50 mL of LB liquid medium containing the corresponding antibiotic (50 μg / ml), culture with shaking at 37 ° C for about 2 h until the OD600 reaches 0.6, add IPTG to induce, the final concentration is 0.5 mM, and induce at 18 ° C Cultivate for 16-18h. After the cultivation, the culture solution was centrifuged at 12,000 rpm for 2 minutes to collect the bacterial cells, the supernatant was discarded, a...
Embodiment 3
[0063] Example 3: Pretreatment of immobilized materials
[0064] First, use distilled water to clean the immobilized carrier: amino resin HA, epoxy resin HFA and Fe 3 o 4 Soak in distilled water for 3 hours, stirring from time to time, repeat 3 times. Then filter under reduced pressure to get the filter cake.
[0065] Amino resin HA and Fe 3 o 4 Need to use glutaraldehyde cross-linking activation, HA and Fe 3 o 4 Use 0.4% glutaraldehyde for 3 hours with continuous stirring, then filter under reduced pressure to get the filter cake, and wash it with distilled water for 3 times.
[0066] The cleaned epoxy resin HFA and activated HA, Fe 3 o 4 Use 0.5% polyethyleneimine (PEI), polyethylene glycol (PEG) and ethanolamine (EA) calcium bicarbonate solutions to soak for 3 hours to wrap, the reaction temperature is 20°C-40°C, and the stirring speed is 100rpm -200rpm. Then the filter cake was obtained by filtration under reduced pressure, and washed 3 times with distilled water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com