Recombinant bacterium expressing D-threonine aldolase and construction method and application of recombinant bacterium
A technology of threonine aldolase and recombinant bacteria, applied in the field of enzyme engineering, can solve the problems of few research reports, and achieve the effects of strong stereoselectivity, environmental friendliness, and good application and development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Screening D-threonine aldolase based on gene mining technology of probe enzyme sequence
[0028] According to the gene sequence of D-threonine aldolase (Alcaligenesxylosoxydans) which has been reported to have aldehyde condensation into p-thymphenylphenylserine, use this sequence as a probe to search in the NCBI database and compare and analyze it by BLAST, find the same For candidate enzyme genes with 40% to 70% homology in the probe sequence, select enzyme genes with high sequence consistency, and ensure that the source strains of these enzyme genes are different from the probe species, and then design primers based on the retrieved gene sequences , using PCR amplification to obtain the DNA encoding these enzymes, and they are cloned and expressed, and finally by screening the activity and stereoselectivity of the target substrate (p-thiamphenicol benzaldehyde), high activity and high selectivity can be obtained. Sexual D-threonine aldolase.
Embodiment 2
[0029] Embodiment 2: Cloning of D-threonine aldolase gene
[0030] The "one-step cloning method" (homologous recombination) was used to construct the recombinant plasmid pET28a-ApDTA.
[0031] (1) First use nutrient broth agar medium (peptone 10.0g, beef extract 3.0g, NaCl 5.0g, agar 15.0g, distilled water 1.0L, pH 7.0) to activate and rejuvenate the above-mentioned Leucobacterium skin type at 25°C . Then, after the colony grows, the single colony is inserted into the liquid medium for cultivation. After the cells were obtained by centrifugation, the bacterial genome DNA kit was used to extract the genomic DNA of Achromobacter piformis.
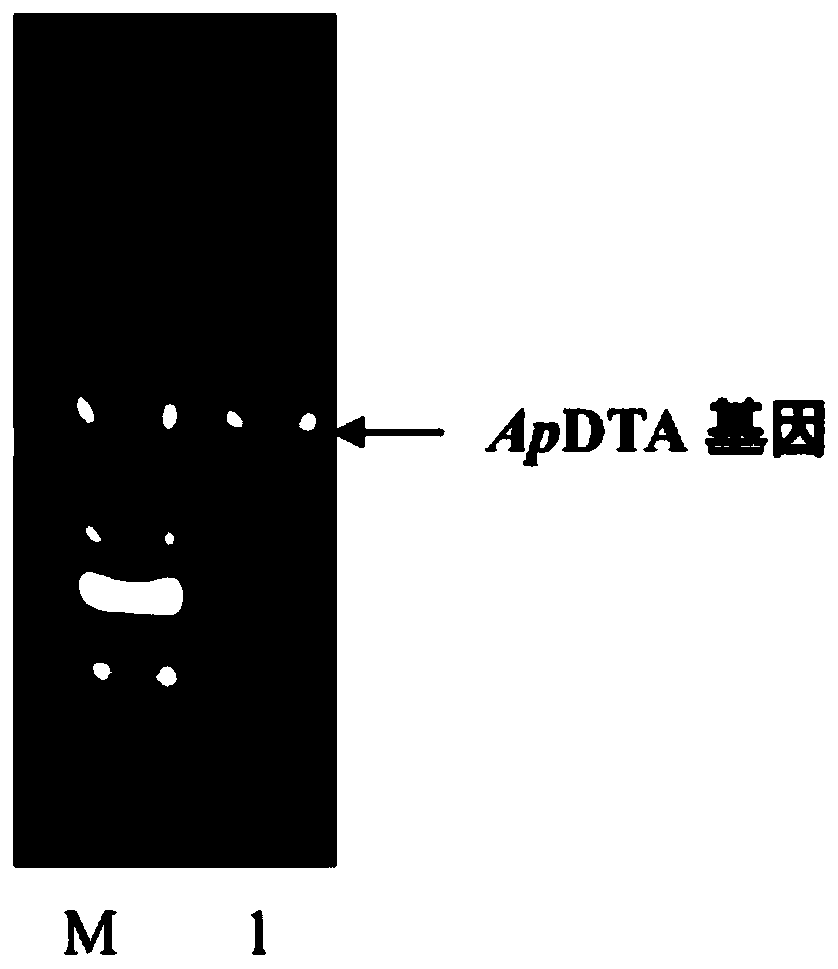
[0032] (2) Design primers according to the reported D-threonine aldolase gene (upstream primer: CAGCAAATGGGTCGC GG ATCC ATGTCCCAGGAAGTCATACGCG, downstream primer: TGCGGCCGCAAGCTT GTC GAC TCAGCGCGAGAAGCCGCG, where GGATCC is the BamHI restriction site and GTCGAC is the SalⅠ restriction site), PCR was performed using the genomic DNA of A...
Embodiment 3
[0033] Example 3: Construction and cultivation of recombinant Escherichia coli BL21(DE3) / pET28a-ApDTA
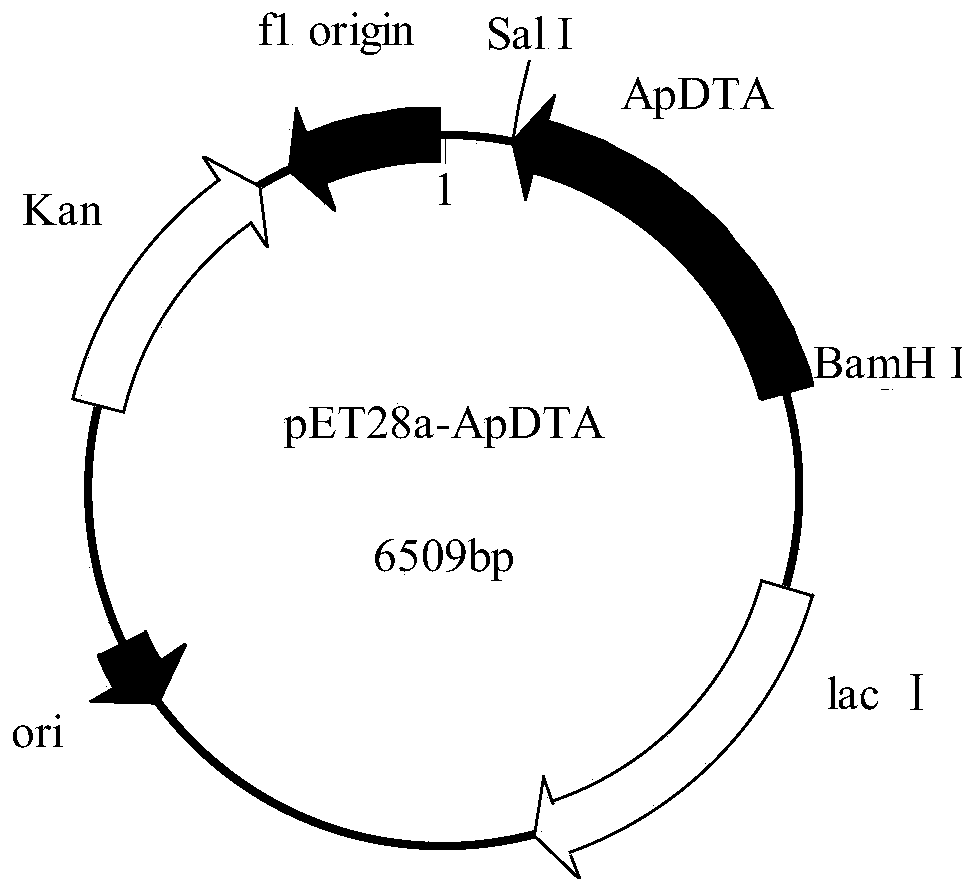
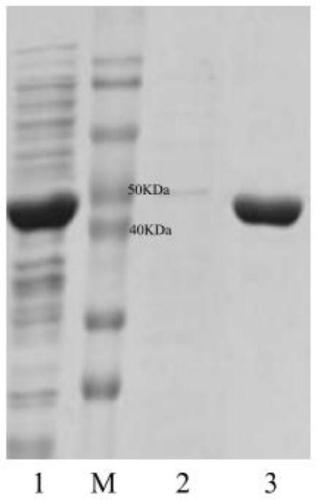
[0034] Plasmid pET28a was double digested with restriction endonucleases BamH I and Sal I in a water bath at 37°C for 4 hours, identified by agarose gel electrophoresis, and the target fragment was purified and recovered using an agarose DNA recovery kit. At 37°C, use the recombinase in the recombination kit to connect the gene ApDTA with the digested plasmid pET28a to obtain the recombinant expression vector pET28a-ApDTA ( figure 2 ). Heat the constructed recombinant expression vector pET28a-ApDTA into Escherichia coli BL21(DE3) competent, coat a solid plate containing kanamycin-resistant LB, and perform colony PCR verification after overnight culture. The positive clone is the gene Recombinant engineering bacteria E.coli BL21(DE3) / pET28a-ApDTA. Pick positive clones and culture them overnight in LB medium, transfer them to fresh LB medium the next day at 2% inoculum size...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com