Preparation of CO2 electro-reduction catalyst, catalyst and application
A CO2 and catalyst technology, applied in the field of single-atom catalyst preparation, can solve the problems of complex preparation method, large environmental pollution, and waste acid generation, and achieve the effects of simple synthesis method, high catalytic activity, and excellent catalytic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
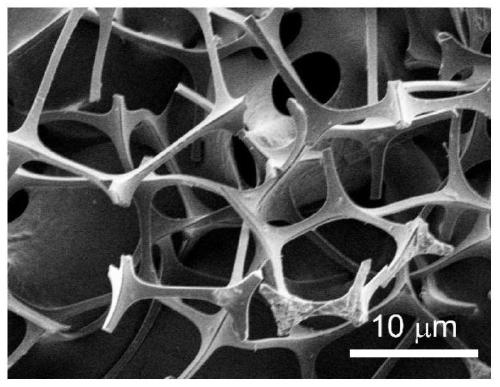
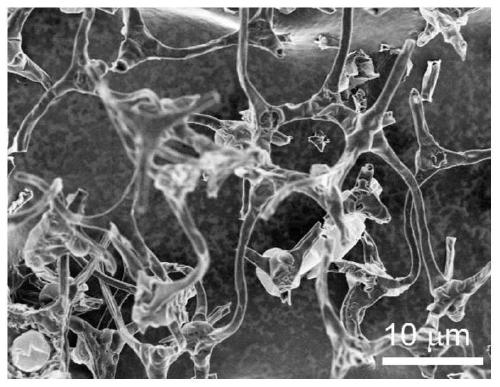
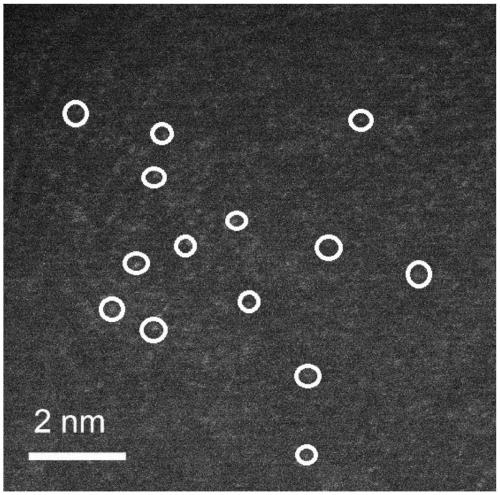
[0031] 1. Preparation of graphene airgel
[0032] (1) Disperse 20mg graphene into 10mL deionized water, add 280uL (0.012mM) of nickel chloride hexahydrate and 15uL of 30wt% hydrogen peroxide, after fully stirring, 60mg of melamine foam is immersed in the above-mentioned mixed solution, and then Dry at 70°C in a vacuum box, repeat dipping-drying until the mixed solution is completely absorbed into the melamine foam, and finally the graphene airgel precursor is obtained.
[0033] (2) Put the graphene airgel precursor into a quartz boat, and under the protection of inert gas Ar, heat up from room temperature to the pyrolysis temperature at a heating rate of 5°C / min, and pyrolyze at a high temperature of 900°C for 1 hour, Graphene airgel is obtained after natural cooling.
[0034] 2. Electrochemical test conditions
[0035] The loading capacity of the catalyst material on the carbon cloth electrode is 0.5 mg / cm 2 , the electrolyte is 0.5M KHCO 3 (pH=7.3), CO 2 Flow rate is 5c...
Embodiment 2
[0039] 1. Preparation of graphene airgel
[0040] (1) Disperse 20mg graphene into 10mL deionized water, add 280uL (0.012mM) of nickel chloride hexahydrate and 15uL of 30wt% hydrogen peroxide, after fully stirring, 60mg of melamine foam is immersed in the above-mentioned mixed solution, and then Dry at 70°C in a vacuum box, repeat dipping-drying until the mixed solution is completely absorbed into the melamine foam, and finally the graphene airgel precursor is obtained.
[0041](2) Put the graphene airgel precursor into a quartz boat, and under the protection of inert gas Ar, heat up from room temperature to the pyrolysis temperature at a heating rate of 5°C / min, and pyrolyze at 800°C for 1 hour. Graphene airgel is obtained after natural cooling.
[0042] 2. Electrochemical test conditions
[0043] The loading capacity of the catalyst material on the carbon cloth electrode is 0.5 mg / cm 2 , the electrolyte is 0.5M KHCO 3 (pH=7.3), CO 2 Flow rate is 5cm 3 / min, room tempera...
Embodiment 3
[0047] 1. Preparation of graphene airgel
[0048] (1) Disperse 20mg graphene into 10mL deionized water, add 280uL (0.012mM) of nickel chloride hexahydrate and 15uL of 30wt% hydrogen peroxide, after fully stirring, 60mg of melamine foam is immersed in the above-mentioned mixed solution, and then Dry at 70°C in a vacuum box, repeat dipping-drying until the mixed solution is completely absorbed into the melamine foam, and finally the graphene airgel precursor is obtained.
[0049] (2) Put the graphene airgel precursor into a quartz boat, and under the protection of inert gas Ar, heat up from room temperature to the pyrolysis temperature at a heating rate of 5°C / min, and pyrolyze at a high temperature of 1000°C for 1 hour, Graphene airgel is obtained after natural cooling.
[0050] 2. Electrochemical test conditions
[0051] The loading capacity of the catalyst material on the carbon cloth electrode is 0.5 mg / cm 2 , the electrolyte is 0.5M KHCO 3 (pH=7.3), CO 2 Flow rate is 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com