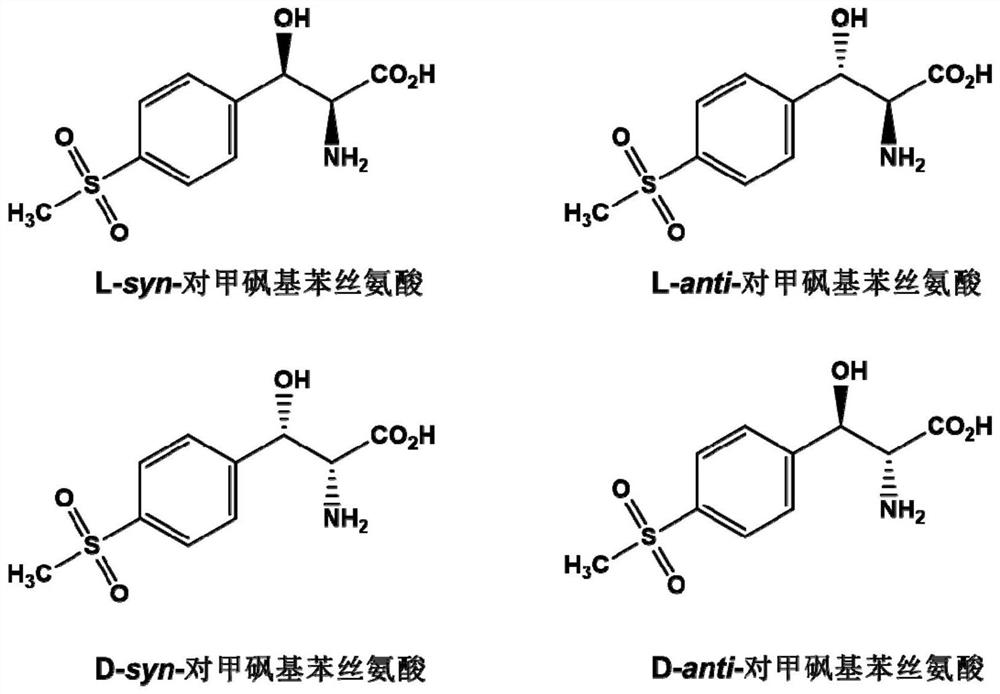
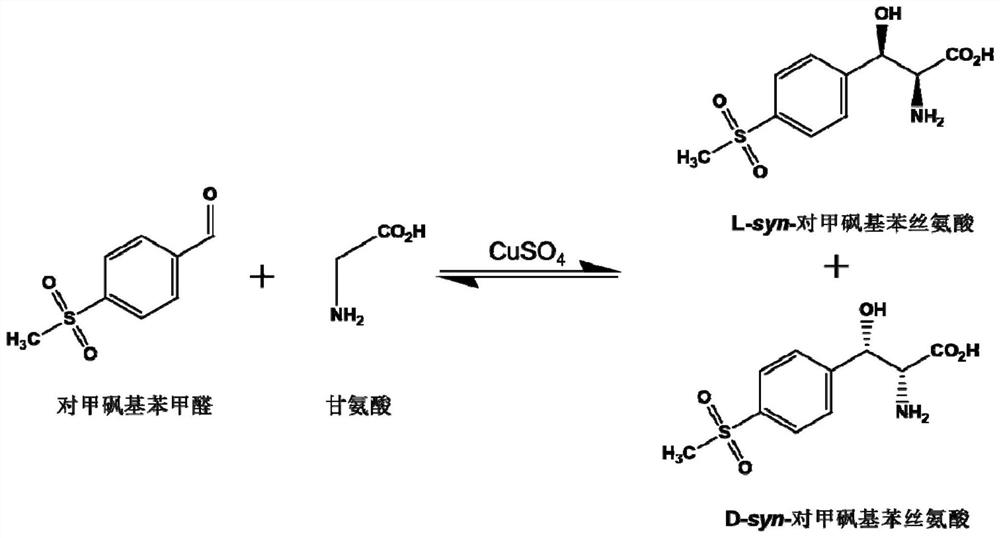
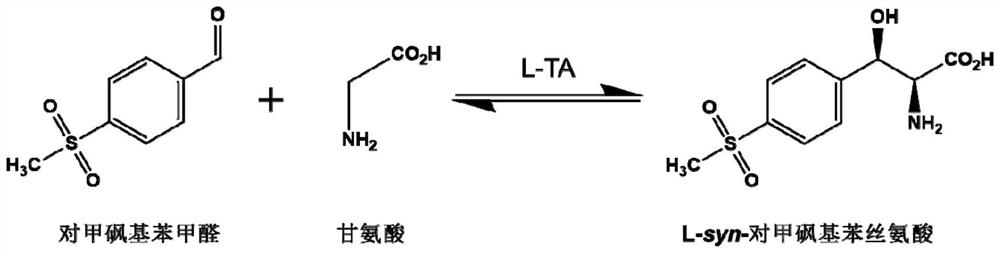
A kind of l-threonine aldolase mutant, gene and method for preparing l-syn-p-thymphenylphenylserine
A methylsulfonyl phenylserine and threonine aldolase technology, which is applied in the field of enzyme engineering, can solve the problems of only 50% theoretical yield, difficult to apply invalid enantiomers, low atom utilization rate, etc., and achieves the optical purity of products. High, high atomic utilization, low pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Construction of wild enzyme engineering bacteria
[0050] Enter keywords such as threoninealdolase in the National Coalition Building Institute (NCBI) database to search and select the amino acid sequence WP_015261381.1 (derived from Desulfitobacterium dichloroeliminans (DdLTA)) and SIS82838.1 (derived from Chryseobacterium chaponense (CcLTA)), WP_016204489.1 (derived from Bacillus nealsonii (BnLTA)), WP_023973138.1 (derived from Clostridium beijerinckii (CbLTA)), WP_077361571.1 (derived from Clostridium saccharoperbutylacetonicum (CsLTA)), 919 (derived from WP4969 in Cellulosilyticum sp.I15G10I2(CpLTA)). The six amino acid sequences were converted into nucleotide sequences according to the codon preference of Escherichia coli (the nucleotide sequences are shown in SEQ ID No. 1-6). The six nucleotide sequences were fully synthesized by chemical method (Anhui General Biology), and integrated between the multiple cloning sites BamH I and Hind III of the exp...
Embodiment 2
[0051] Embodiment 2: the construction of mutant enzyme
[0052] 1. Activation of Engineering Bacteria and Plasmid Extraction
[0053] All engineering bacteria (constructed and obtained in Example 1) were activated and cultivated using LB medium, and the formula was: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, 115 Sterilize at ℃ for 30min, and set aside. The solid medium is LB medium with 2% agar added.
[0054] The preserved engineered bacteria glycerol tube was inserted into a test tube containing 10 mL of LB medium, and cultured at 37°C and 200 rpm for 12 hours. After obtaining the cultured cells, plasmid extraction was carried out according to the operating instructions of the Axygen plasmid extraction kit. The resulting plasmid can be directly used for point mutation or stored at -20°C for a long time.
[0055] 2. Gene site-directed mutation
[0056] The gene mutation adopts the method of whole plasmid PCR to ob...
Embodiment 3
[0071] Embodiment 3: the preparation of the culture of thalline and crude enzyme liquid
[0072] 1. Bacteria culture
[0073] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 115°C for 30min, ready for use.
[0074] After the engineering bacteria containing the L-threonine aldolase gene were activated by streaking on a plate, a single colony was picked and inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer 2% of the inoculum into 50 mL of fresh LB liquid medium also containing 50 μg / ml Kan, culture with shaking at 37 °C until the OD600 reaches about 0.6, add IPTG to a final concentration of 0.5 mM, and induce culture at 28 °C 10h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, and the bacterial cells were collected, and stored in a -80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com