L-threonine aldolase mutant R318M and application thereof
A technology of threonine aldolase and mutant, which is applied in the field of L-threonine aldolase mutant R318M, can solve the problems of low diastereoselectivity, hindering application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
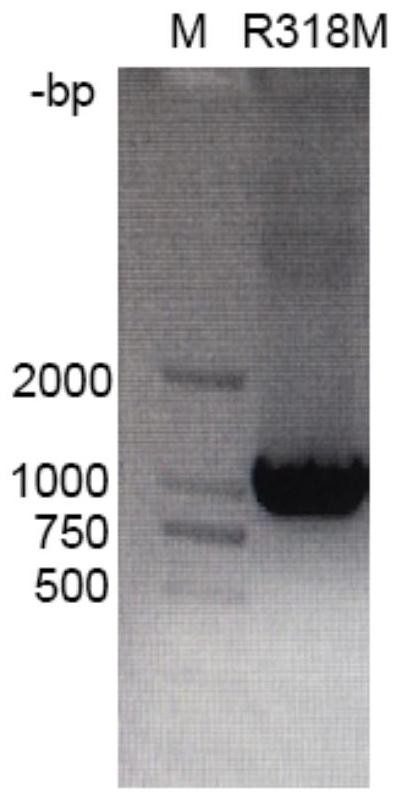
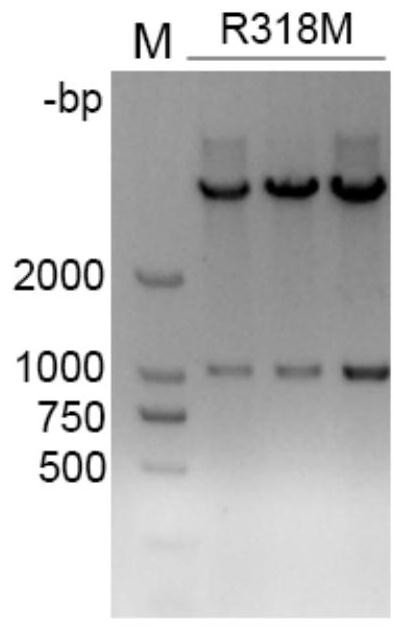
[0042] Example 1. Preparation of L-threonine aldolase R318M mutant
[0043] 1. Design of L-threonine aldolase mutants
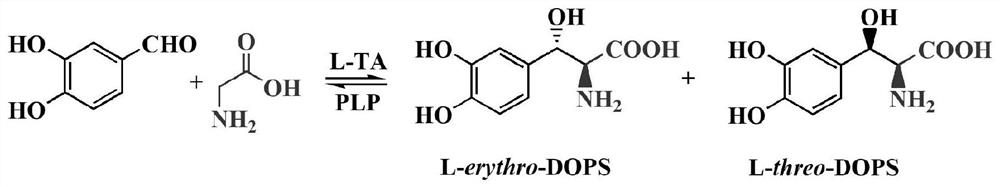
[0044] The wild-type L-threonine aldolase gene (L-TA gene, SEQ ID NO: 2) was isolated from feces samples of healthy black bears in the Sichuan Black Bear Protection and Incubation Base in our laboratory. The L-TA gene The full length of the open reading frame is 1032bp, and the encoded L-threonine aldolase consists of 343 amino acids. The isolation and cloning process of the gene is described in the patent application text with the application number 2019109657806, the publication number CN110592058A, and the invention title "threonine aldolase, its coding gene and its application in the biosynthesis of droxidopa" , which is incorporated herein by reference in its entirety.
[0045] The amino acid sequence (343aa) of the wild-type L-threonine aldolase is:
[0046] MYSFKNDYSEGAHPRILETLLRTNLEQCEGYGKDTYCEEAENLIKNKLNNESIEVHFISGGTQTNLIAISAFLRPHEGVISADTGHIFVNEAG...
Embodiment 2
[0093] Example 2. Utilize L-TA R318M mutant to synthesize droxidopa and detect de value
[0094] 1. Synthesis of droxidopa
[0095] The substrate solution was prepared in 10 mM PBS at pH 7.4, containing 1 M glycine, 60 mM 3,4-dihydroxybenzaldehyde and 50 μM pyridoxal 5-phosphate. Add 20 U of the L-TA R318M enzyme prepared in Example 1 to each ml of the substrate solution, mix well, place at 25° C. for 2 h, and then obtain a sample by ultrafiltration (10 kDa, 4000×g, 30 min).
[0096] 2. Detection of droxidopa and calculation of de value
[0097] The sample obtained after ultrafiltration in step 1 was diluted 10 times to achieve better HPLC separation effect. The amount of droxidopa (L-threo-DOPS) and its diastereoisomer (L-erythro-DOPS) in the sample was detected by HPLC. HPLC instrument model: Agilent 1260. Parameter settings: Chromatographic column: COSMOSIL 5C18-MS 4.6×150mm; wavelength 280nm, column temperature 30°C; mobile phase: 90% 0.1% (w / v, g / mL) 1-heptanesulfonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com