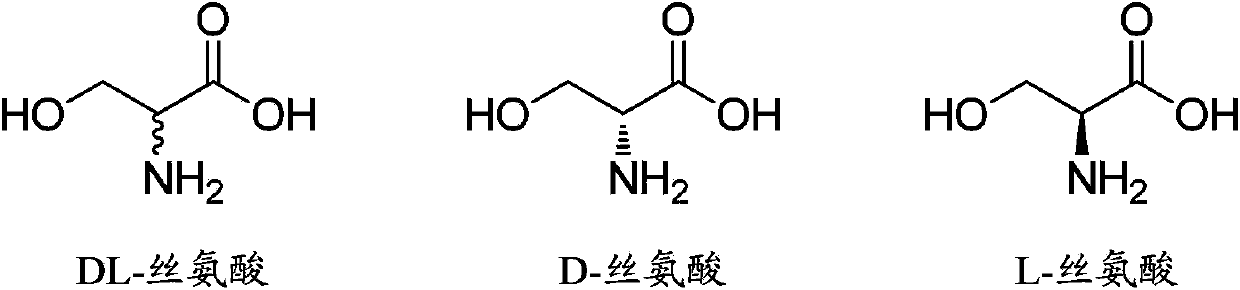
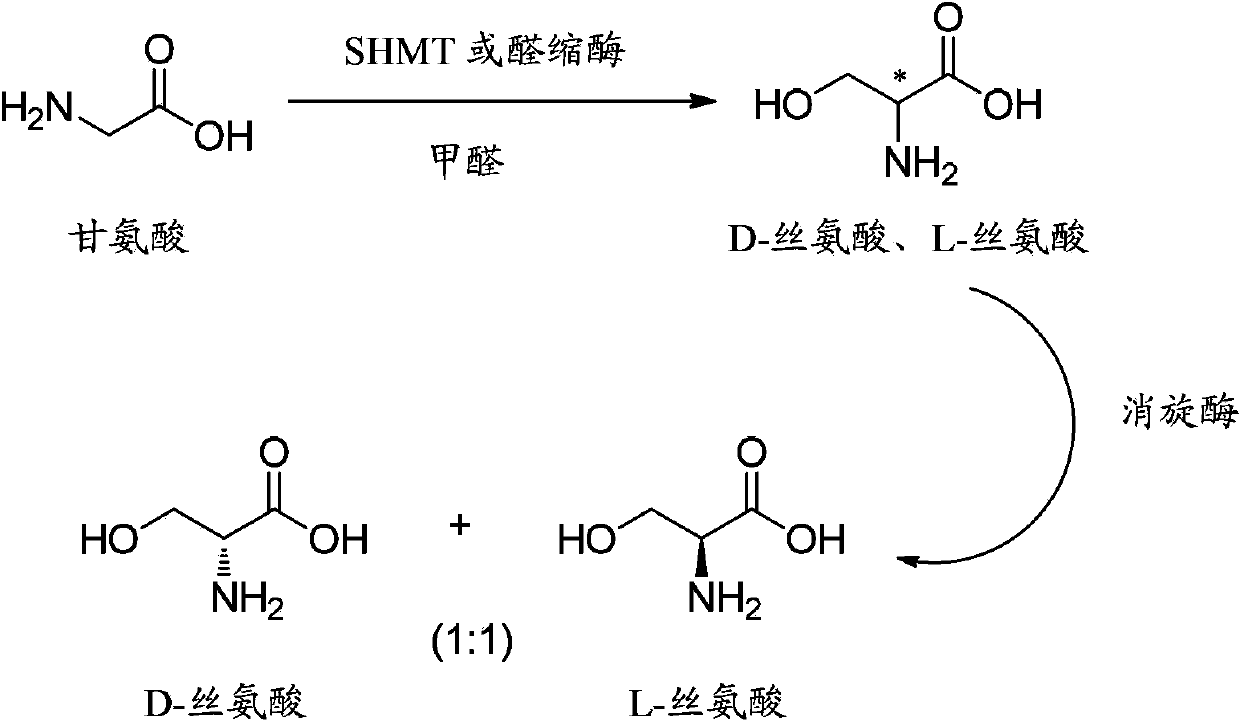
Method for synthesizing DL-serine through enzyme catalysis method
A technology of enzymatic catalysis and serine, which is applied in the field of enzymatic catalysis to synthesize DL-serine, can solve the problems of harsh reaction conditions, unfriendly environment, and high cost of raw materials, and achieve the effects of short production cycle, low pressure on environmental protection, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 15 g of the substrate glycine with an electronic balance, add 1.5 gram of wet thallus containing serine hydroxymethyltransferase and 0.5 g of wet thallus containing amino acid racemase, join in a reaction kettle with deionized water, and then Add 30 mg of pyridoxal phosphate and 20 mg of tetrahydrofolic acid, and adjust the volume to 1000 mL with deionized water; control the temperature at 30°C through water bath circulation; add 37% formaldehyde aqueous solution, and control the pH value to 7.5 through 4M sodium hydroxide aqueous solution ; Stirring speed 200rpm, HPLC detection glycine conversion rate reached 99.1%, the reaction time was 6h.
[0043] The conversion solution is filtered or centrifuged to remove bacteria, and then ultrafiltered to remove protein, decolorized by activated carbon, concentrated under reduced pressure, crystallized, and dried in an oven at 70°C overnight to obtain white crystalline powder, odorless and sweet, and the obtained serine pro...
Embodiment 2
[0045] Weigh 30 g of the substrate glycine with an electronic balance, add 2.5 g of wet cells containing D-threonine aldolase and 1.5 g of wet cells containing amino acid racemase, and add them to the reaction kettle with deionized water , then add 40 mg of pyridoxal phosphate and 150 mg of anhydrous magnesium sulfate, and use deionized water to adjust the volume to 1000 mL; control the temperature at 40 °C through water bath circulation; add 37% formaldehyde aqueous solution, and control the pH with 4M sodium hydroxide aqueous solution The value is 6.8; the stirring speed is 220rpm, and after the reaction time is 17h, the conversion rate of glycine detected by HPLC reaches 99.5%.
[0046] The conversion solution is filtered or centrifuged to remove bacteria, and then ultrafiltered to remove protein, decolorized by activated carbon, concentrated under reduced pressure, crystallized, and dried in an oven at 70°C overnight to obtain white crystalline powder, odorless and sweet, a...
Embodiment 3
[0048] Weigh 60g of the substrate glycine with an electronic balance, add 100mL of bacteriostasis solution containing serine hydroxymethyltransferase, add it to the reaction kettle with deionized water, and then add 75mg of pyridoxal phosphate and 40mg of tetrahydrofolate , use deionized water to set the volume to 1000mL; through water bath circulation, control the temperature at 50°C; add 37% formaldehyde aqueous solution, and control the pH value to 8.0 through 4M sodium hydroxide aqueous solution; stir at 300rpm, react for 26h, and detect by HPLC The conversion rate of glycine reached 98%, and the specific optical rotation of the reaction solution measured by the polarimeter was +14.5°; at this time, 3 g of wet bacteria containing amino acid racemase was added to the reaction solution to continue the reaction, and the conversion rate of glycine reached 99.7 after 12 hours. %, the specific rotation of the reaction solution is 0.03.
[0049] The conversion solution is filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com