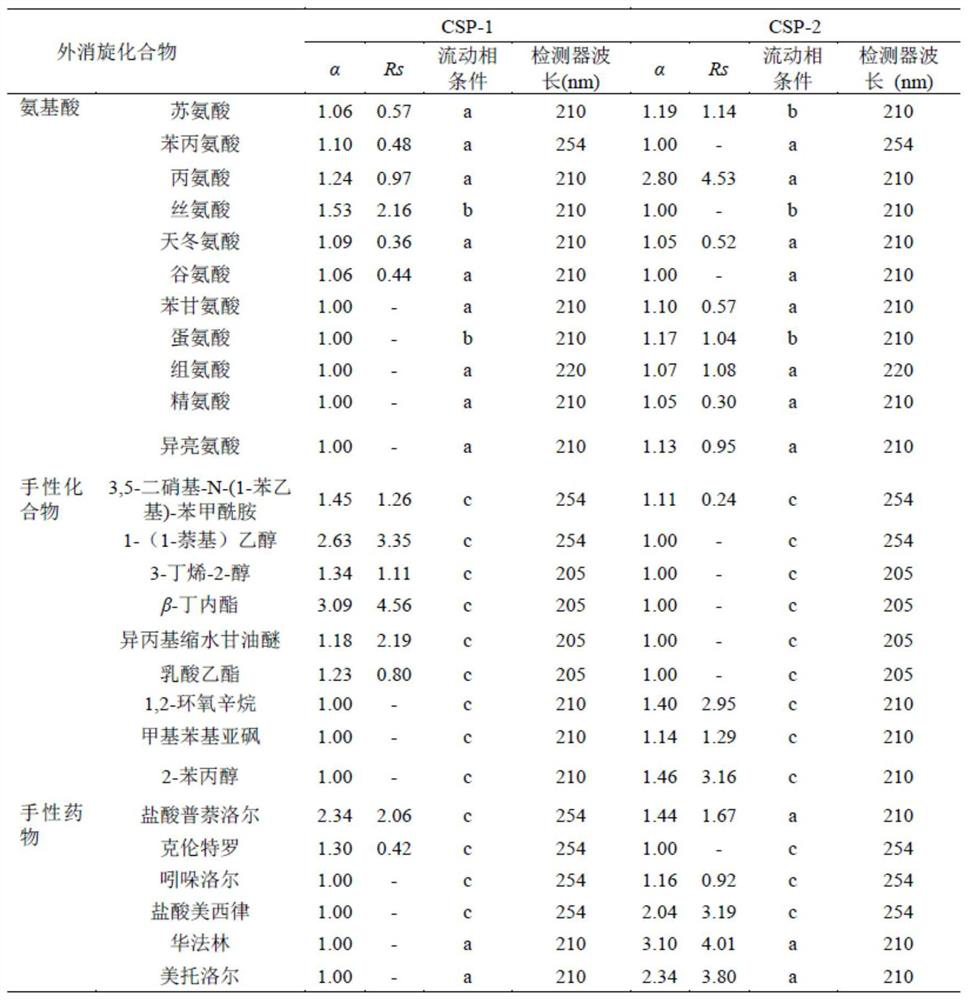
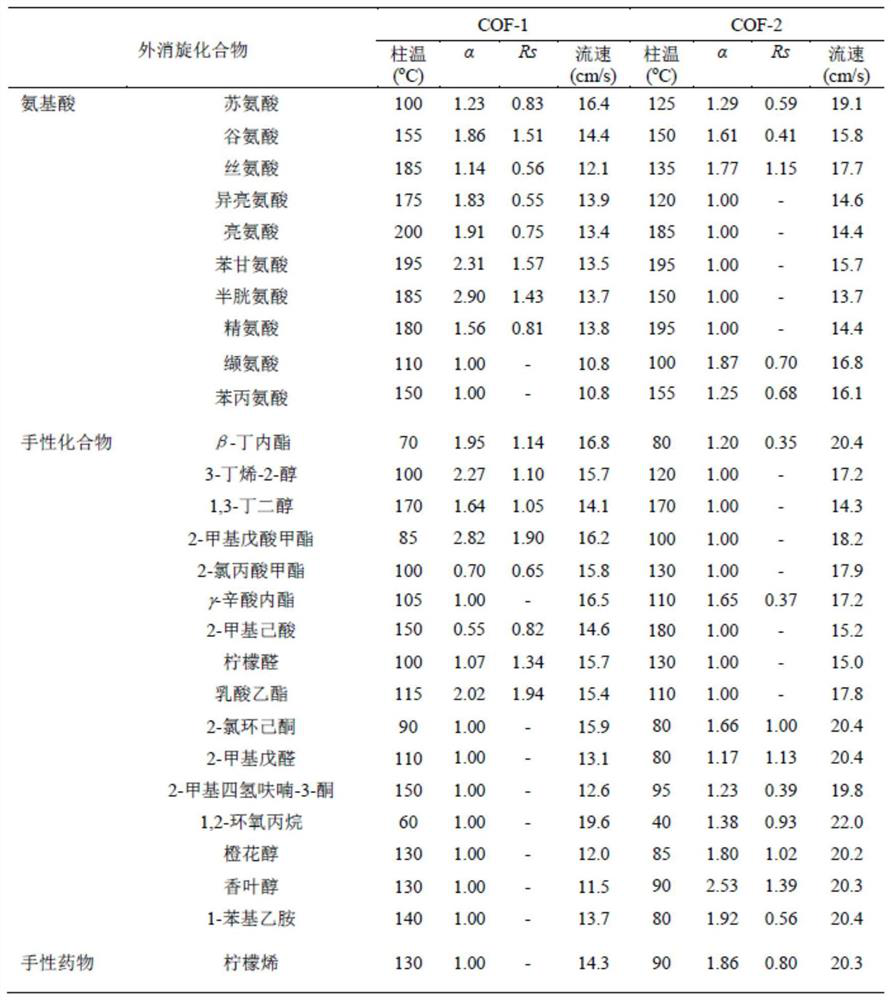
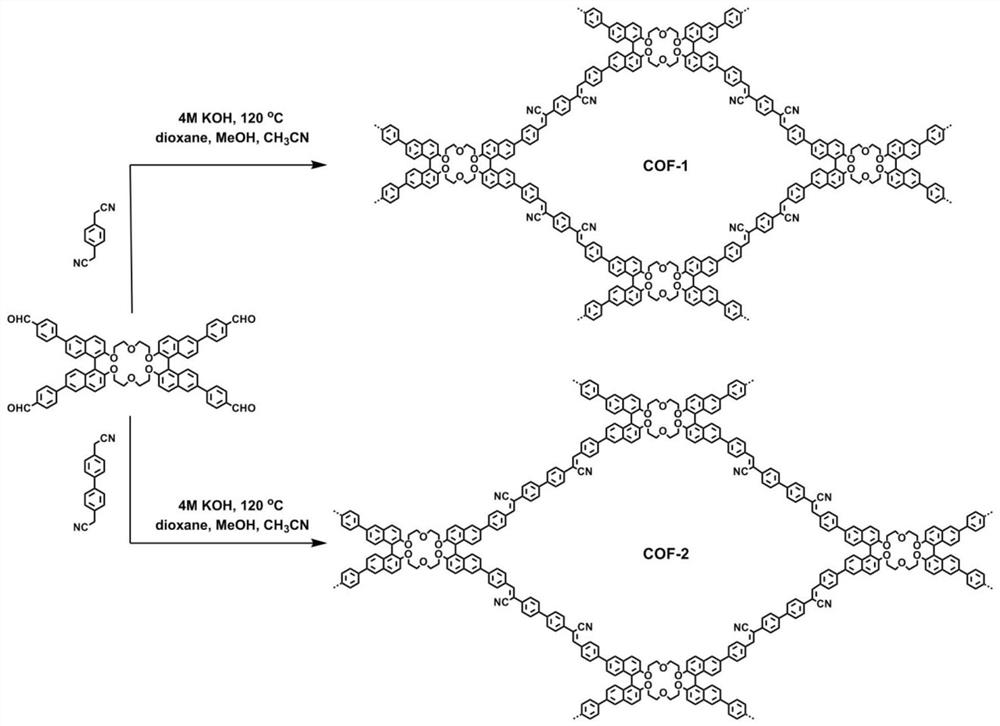
Application of C=C bond connection-based COF material in preparation of chiral chromatographic stationary phase
A chiral stationary phase and chiral chromatography technology, applied in the application field of making chiral chromatographic stationary phases, can solve the problems of strict conditions of use, single type of separation substrate, etc., to broaden design ideas, high chiral selectivity, good The effect of chiral separation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] In one embodiment of the present invention, the preparation method of COF material based on C=C bond connection, the steps are as follows:
[0061] Mix crown ether functionalized bis-naphthalene tetraaldehyde-based monomers, terephthalonitrile or 4,4'-biphenyldiacetonitrile, dioxane, and methanol, heat to the reaction temperature under vacuum-sealed conditions, and perform the reaction. After the reaction, the mixture was cooled to room temperature and filtered to obtain a powdery solid, which was then washed and dried to obtain COF-1 or COF-2.
[0062] In one embodiment of the present invention, based on the preparation method of C=C bond-linked COF materials, an exemplary implementation condition is given:
[0063] Crown ether functionalized bis-naphthyl tetraaldehyde monomer (30 mg, 0.027 mmol), terephthalonitrile (8.3 mg, 0.053 mmol) or 4,4'-biphenyl diacetonitrile (12.4 mg, 0.053 mmol), Dioxane (0.5 mL), methanol (0.5 mL) were mixed in a 10 mL Schlenk tube. The S...
Embodiment 1
[0085] Preparation of high stability COF-1:
[0086] In a 10 mL Schlenk tube, 30 mg of a crown ether functionalized bis-naphthyl backbone tetraaldehyde monomer, 8.3 mg of terephthalonitrile, 0.5 mL of dioxane, and 0.5 mL of methanol were mixed. The Schlenk tube was vacuum sealed and heated to 120°C for a reaction time of 4 days. The mixture was cooled to room temperature and filtered to obtain a powdery solid, which was washed three times with deionized water, tetrahydrofuran and ether, and dried under vacuum at 60°C overnight to obtain 28 mg of light yellow COF-1 powder.
Embodiment 2
[0088] Preparation of high stability COF-2:
[0089] In a 10 mL Schlenk tube, 30 mg of the tetraaldehyde monomer of the crown ether functionalized bis-naphthalene backbone, 12.4 mg of 4,4'-biphenyldiacetonitrile, 0.5 mL of dioxane, and 0.5 mL of methanol were mixed. The Schlenk tube was vacuum sealed and heated to 120°C for a reaction time of 4 days. The mixture was cooled to room temperature, filtered to obtain a powdery solid, washed three times with deionized water, tetrahydrofuran and diethyl ether, and dried under vacuum at 60°C overnight to obtain 29 mg of light yellow COF-2 powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com