Method for preparing chiral amino acid tetrazole compound
A technology of amino acid tetrazole and compound, which is applied in the field of preparation of chiral amino acid tetrazole compounds, can solve the problems of racemization and low yield, and achieve the effects of simple operation, easy separation and recovery, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
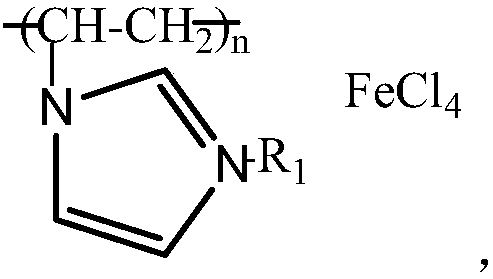
[0034] Preparation of imidazole polymer iron salt ionic liquid
[0035] Add vinylimidazole (5mmol) into a three-necked flask, then add chlorobutane (5mmol), and stir at 40°C for 12h to obtain 1-vinyl-3-n-butynylimidazolium chloride.
[0036] Weigh 0.94g of 1-vinyl-3-n-butyrylimidazolium chloride (5mmol) and dissolve it in 5mL of methanol, add 0.05g of dibenzoyl peroxide, stir at 60°C for 15h, cool down to 50°C, add 0.85g anhydrous FeCl 3 (5 mmol), stirred at 50° C. for 0.5 h, and spin-dried the organic solvent to obtain a yellow solid, which was vacuum-dried at 50° C. to obtain an imidazole polymer iron salt ionic liquid.
Embodiment 2
[0038] Preparation of imidazole polymer iron salt ionic liquid
[0039] Add vinylimidazole (5mmol) into a three-necked flask, then add chlorobutane (15mmol), and stir at 80°C for 5h to obtain 1-vinyl-3-n-butynylimidazolium chloride.
[0040] Weigh 0.94g of 1-vinyl-3-n-butyrylimidazolium chloride (5mmol) and dissolve it in 45mL of methanol, add 0.01g of dibenzoyl peroxide, stir at 90°C for 6h, cool down to 30°C, add 0.42g anhydrous FeCl 3 (2.5 mmol), stirred at 30° C. for 3 h, and spin-dried the organic solvent to obtain a yellow solid, which was vacuum-dried at 50° C. to obtain the imidazole polymer iron salt ionic liquid.
Embodiment 3
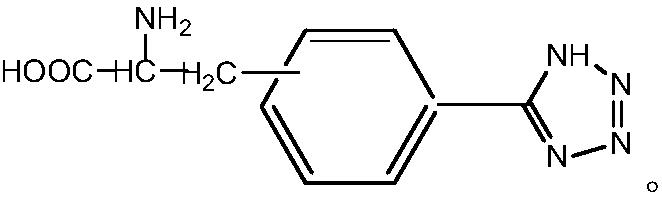
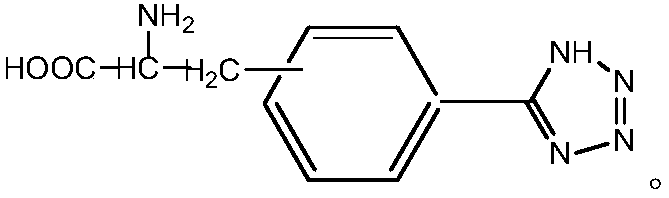
[0042] Preparation of compound 5-(D-3'-alanine)phenyltetrazolium:
[0043] Add 0.25g D-3-cyanophenylalanine (2.5mmol) successively in 250mL three-necked flask, 0.32g sodium azide (5.5mmol), the 0.07g imidazole polymer iron salt ionic liquid that embodiment 1 prepares and dry 5mL DMF, install a stirrer, a reflux condenser, and a thermometer, raise the temperature to 120°C while stirring, and react at a constant temperature for 24h. Cool, filter and separate the catalyst, wash the catalyst three times with 5 mL of ethyl acetate, dry it and save it for reuse, adjust the pH of the separation liquid to neutral with 6N hydrochloric acid, extract and separate the liquid with 30 mL of ethyl acetate, take the upper organic phase, wash it with 20 mL of water for two After three times, the organic solvent was spin-dried to obtain a white solid, which was dried in vacuum at 50°C and weighed. The yield was 80%, and the chiral purity was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com