A kind of preparation method of high chiral purity lactam compound
A technology for the purity of lactam compounds, which is applied in the field of preparation of greatly improving the chiral purity of lactam compounds, can solve problems such as poor atom economy, unfavorable scale-up production, and high hydrogen pressure, so as to reduce production costs and improve chiral selection high efficiency and atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
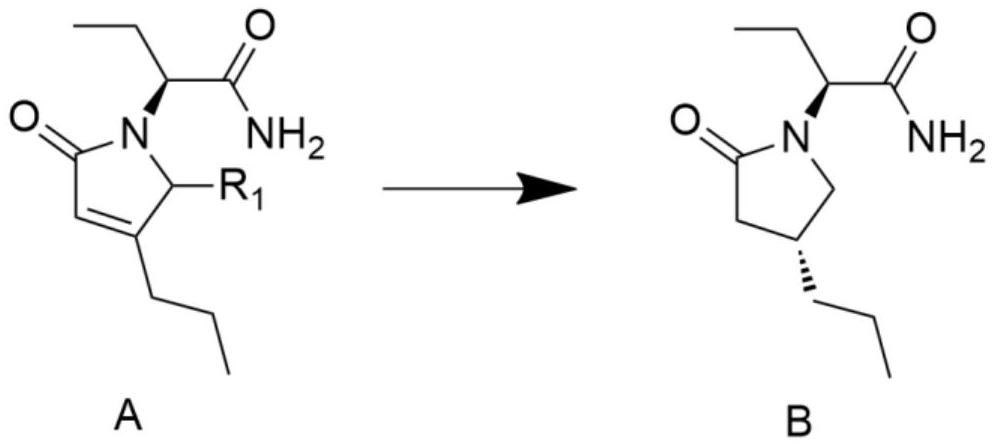
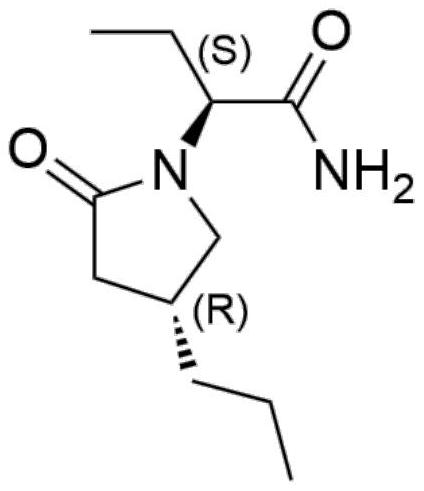
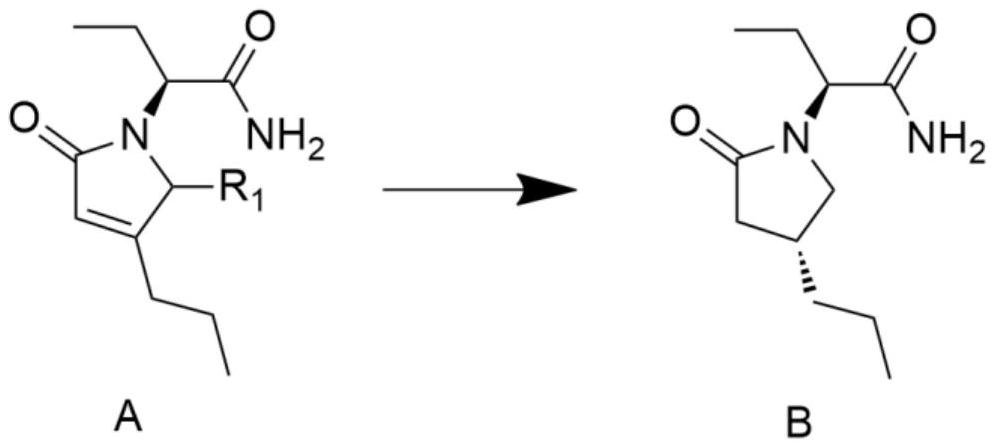
[0028] The preparation method of (2S)-2-((4R)-2-oxo-4-n-propyl-1-pyrrolidinyl)butyramide comprises the steps:
[0029] Add 50ml of water and 200ml of methanol into a 500ml reaction vessel, add the heavy metal catalyst 5% palladium calcium carbonate (0.1g), stir for 10-30 minutes, add the reaction raw material molecular formula A (R1 is hydrogen), (2S)-(2-oxo Substitute-4-propyl-2,5-dihydro-1-pyrrolyl)butyramide 20g, then add hydrogen into the reaction vessel, then react at -10°C to 0°C for 20 to 40 hours, after the reaction is completed, the Adjust the temperature to 0-30 degrees, spin off the organic solvent at 30-40 degrees, add 100ml of isopropyl acetate for extraction, then, the organic phase is dried, filtered with suction, concentrated to obtain 16g of crude product B, and then the crude product B is mixed with 16ml of isopropyl acetate Propyl ester was recrystallized to obtain product B14.2g with a yield of 70.2% and a de value of 95.4%.
Embodiment 2
[0031] The preparation method of (2S)-2-((4R)-2-oxo-4-n-propyl-1-pyrrolidinyl)butyramide comprises the steps:
[0032] Add 100 ml of isopropanol to a 250 ml reaction vessel, add 1 g of heavy metal catalyst 10% palladium carbon, stir for 10 to 30 minutes, add the reaction raw material molecular formula A (R1 is hydroxyl), (2S)-(2-oxo-4-propane Base-2,5-dihydro-5-hydroxyl-1-pyrrolyl)butanamide 10g, then add hydrogen into the reaction vessel, then react at a constant temperature of -30~-20°C for 20~40 hours, after the reaction is completed, the temperature Adjust to 0-30°C, spin off the organic solvent at 30-40°C, add 50ml and 50ml of isopropyl acetate for extraction, then, the organic phase is dried, suction filtered and concentrated to obtain 8g of crude product B, and then the crude product B is mixed with 16ml of isopropyl acetate Propyl ester was recrystallized to obtain 6.8 g of product B with a yield of 72.4% and a de value of 96.2%.
Embodiment 3
[0034] The preparation method of (2S)-2-((4R)-2-oxo-4-n-propyl-1-pyrrolidinyl)butyramide comprises the steps:
[0035] Add 200ml of methanol to a 500ml reaction vessel, add 4g of heavy metal catalyst 5% palladium calcium carbonate, stir for 10 to 30 minutes, add the reaction raw material formula A (R1 is hydrogen), (2S)-(2-oxo-4-propyl -2,5-dihydro-1-pyrrolyl)butyramide 20g, then add 9g of formic acid into the reaction vessel, then react at a constant temperature at -20°C for 20-30 hours, after the reaction is complete, adjust the temperature to 0-30°C , add saturated aqueous sodium bicarbonate solution to adjust pH = 7-8, spin off the organic solvent at 30-40 degrees, add 100ml of water and 100ml of isopropyl acetate for extraction, then, the organic phase is dried, suction filtered and concentrated to obtain 17g of crude product, Then the crude product was recrystallized with 34ml of isopropyl acetate to obtain 15.4g of the product with a de value of 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com