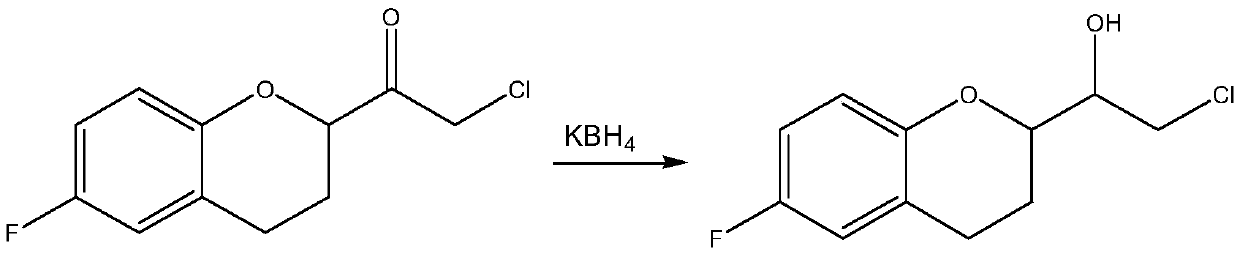
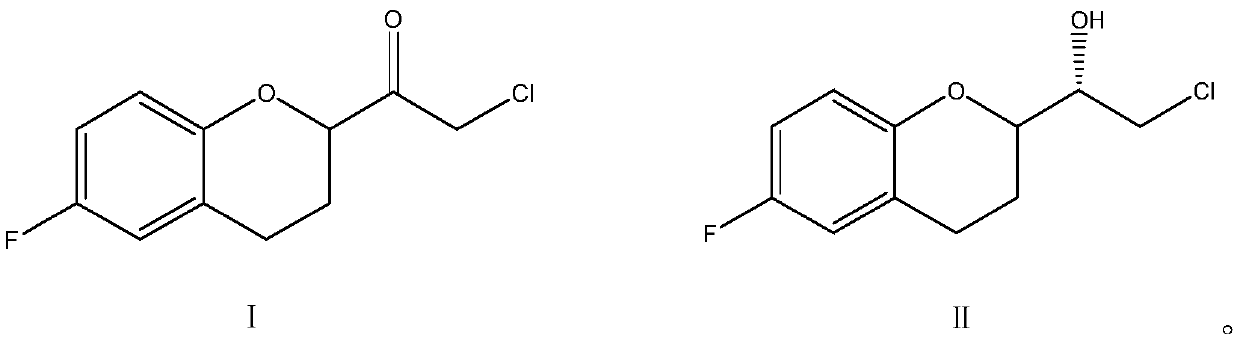
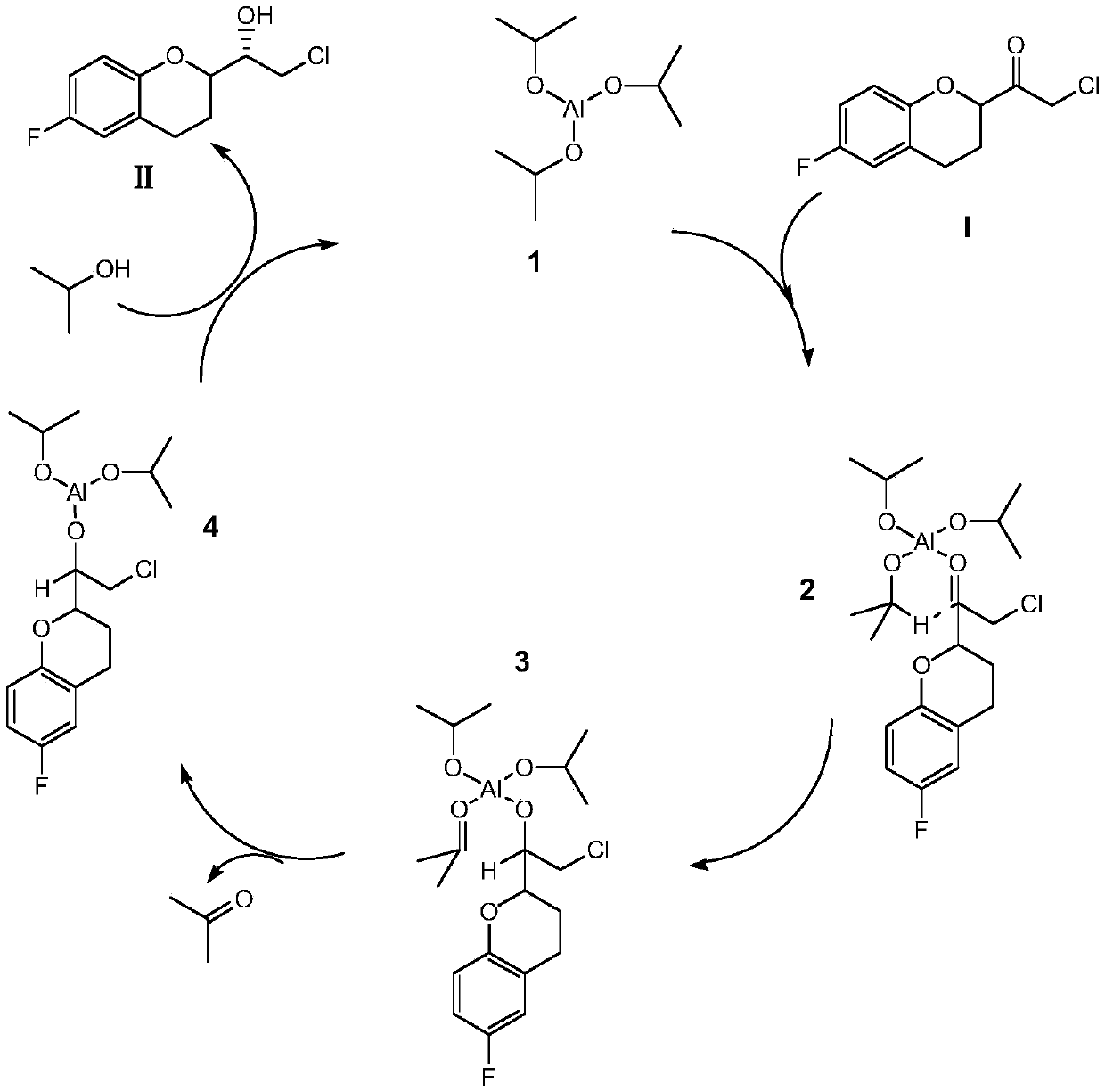
A kind of preparation method of (s)-2-chloro-1-(6-fluoro-1-chroman-2-yl)-ethanol
A chroman and ethanol technology, applied in the directions of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of irrespective of chiral selectivity, low chiral purity of products, difficulty in separation and purification, and the like, Achieve high chiral selectivity, high chiral purity, high yield, and high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take 22.87g (0.1mol) of (S)-(6-fluoro-3,4-dihydro-2H-chroman-2-methanol-2-yl)ethanone chloride into the reaction flask, add 230mL of dichloromethane, stirred at room temperature, then added 30g (0.5mol) isopropanol, continued to stir and mix evenly, then, added 6.0g (0.03mol) aluminum isopropoxide, stirred until uniform. Slowly raise the temperature of the water bath to 30°C, and control the temperature at 30°C to 35°C to keep warm for catalytic reduction reaction for 4 hours. After the reaction, add 5wt% dilute hydrochloric acid to wash with water, let stand to separate the liquid, collect the organic phase, and then After adding water and washing once, the organic phase was dried with anhydrous sodium sulfate for 0.5 hours, filtered, and the collected filtrate was subjected to vacuum distillation to remove the solvent to obtain the corresponding finished product (S)-2-chloro-1-((S)-6-fluoro -1-chroman-2-yl)-ethanol 19.38g, yield 84.03%, content 97.58%, chiral purity 9...
Embodiment 2
[0033]Take 22.87g (0.1mol) of (S)-(6-fluoro-3,4-dihydro-2H-chroman-2-methanol-2-yl)ethanone chloride into the reaction flask, add 250mL of toluene, stirred at room temperature, then added 36g (0.6mol) isopropanol, continued to stir, then added 8.0g (0.04mol) aluminum isopropoxide, stirred until uniform, then slowly raised the temperature to 60°C in a water bath, and controlled the reaction system The temperature is kept at 58°C-60°C for 4 hours of catalytic reduction reaction. After the reaction, add 5wt% dilute hydrochloric acid to wash with water, stand to separate the liquid, collect the organic phase, and then add water to wash once. The organic phase is anhydrous Sodium sulfate was dried for 0.5 hours, filtered, and the collected filtrate was subjected to vacuum distillation to remove the solvent to obtain the corresponding finished product (S)-2-chloro-1-((S)-6-fluoro-1-chroman- 2-yl)-ethanol 20.24g, yield 87.76%, content 98.02%, chiral purity 96.63%.
Embodiment 3
[0035] Take 22.87g (0.1mol) of (R)-(6-fluoro-3,4-dihydro-2H-chroman-2-methanol-2-yl)ethanone chloride into the reaction flask, add 300mL of toluene, stirred at room temperature, then added 24g (0.4mol) isopropanol, continued to stir, then added 4.0g (0.02mol) isobutyl aluminum, stirred until uniform, then slowly raised the temperature to 80°C in a water bath, and controlled the reaction system The temperature was kept at 75°C to 80°C to carry out the catalytic reduction reaction for 3 hours. After the reaction, add 5wt% dilute hydrochloric acid to wash, stand to separate the liquid, collect the organic phase, and then add water to wash once. Add 2g of organic phase to the organic phase dried over sodium sulfate for 0.5 hours, filtered, and the collected filtrate was distilled under reduced pressure to remove the solvent to obtain the corresponding finished product (S)-2-chloro-1-((R)6-fluoro-1-chroman- 2-yl)-ethanol 21.12g, yield 91.6%, content 98.51%, chiral purity 97.63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com